FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
RAYALDEE is a vitamin D3 analog indicated for the treatment of secondary hyperparathyroidism in adult patients with stage 3 or 4 chronic kidney disease and serum total 25-hydroxyvitamin D levels less than 30 ng/mL.
Limitations of Use
RAYALDEE is not indicated for the treatment of secondary hyperparathyroidism in patients with stage 5 chronic kidney disease or in patients with end-stage renal disease on dialysis.
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
- •
- Ensure serum calcium is below 9.8 mg/dL before initiating treatment [see Warnings and Precautions (5.1)].
- •
- Instruct patients to swallow RAYALDEE capsules whole.
- •
- Instruct patients to skip a missed dose and to resume taking the medicine at the next regularly scheduled time. Do not administer an extra dose.
2.2 Starting Dose and Dose Titration
- •
- The initial dose of RAYALDEE is 30 mcg administered orally once daily at bedtime.
- •
- The maintenance dose of RAYALDEE should target serum total 25-hydroxyvitamin D levels between 30 and 100 ng/mL, intact parathyroid hormone (PTH) levels within the desired therapeutic range, serum calcium (corrected for low albumin) within the normal range and serum phosphorus below 5.5 mg/dL.
- •
- Monitor serum calcium, serum phosphorus, serum total 25-hydroxyvitamin D and intact PTH levels at a minimum of 3 months after initiation of therapy or dose adjustment, and subsequently at least every 6 to 12 months.
- •
- Increase the dose to 60 mcg orally once daily at bedtime after approximately 3 months, if intact PTH remains above the desired therapeutic range. Prior to raising the dose, ensure serum calcium is below 9.8 mg/dL, serum phosphorus is below 5.5 mg/dL and serum total 25-hydroxyvitamin D is below 100 ng/mL.
- •
- Suspend dosing if intact PTH is persistently and abnormally low to reduce the risk of adynamic bone disease [see Warnings and Precautions (5.3)], if serum calcium is consistently above the normal range to reduce the risk of hypercalcemia [see Warnings and Precautions (5.1)], or if serum total 25-hydroxyvitamin D is consistently above 100 ng/mL. Restart at a reduced dose after these laboratory values have normalized.
3 DOSAGE FORMS AND STRENGTHS
Extended-release capsules, 30 mcg available as:
- •
- blue oval soft capsules imprinted with “O” in white ink
- •
- White two-piece banded hard capsules imprinted with ”O” and “30” in blue ink
5 WARNINGS AND PRECAUTIONS
5.1 Hypercalcemia
Hypercalcemia may occur during RAYALDEE treatment [see Adverse Reactions (6.1)]. Acute hypercalcemia may increase the risk of cardiac arrhythmias and seizures and may potentiate the effect of digitalis on the heart [see Warnings and Precautions (5.2)]. Chronic hypercalcemia can lead to generalized vascular calcification and other soft-tissue calcification. Severe hypercalcemia may require emergency attention.
Hypercalcemia may be exacerbated by concomitant administration of high doses of calcium containing preparations, thiazide diuretics, or other vitamin D compounds. In addition, high intake of calcium and phosphate concomitantly with vitamin D compounds may lead to hypercalciuria and hyperphosphatemia. In these circumstances, frequent serum calcium monitoring and RAYALDEE dose adjustments may be required. Patients with a history of hypercalcemia prior to initiating therapy with RAYALDEE should be monitored more frequently for possible hypercalcemia during therapy.
Patients should be informed about the symptoms of elevated serum calcium, which include feeling tired, difficulty thinking clearly, loss of appetite, nausea, vomiting, constipation, increased thirst, increased urination, and weight loss.
5.2 Digitalis Toxicity
Hypercalcemia of any cause, including RAYALDEE [see Warnings and Precautions (5.1)], increases the risk of digitalis toxicity. In patients using RAYALDEE concomitantly with digitalis compounds, monitor both serum calcium and patients for signs and symptoms of digitalis toxicity and increase the frequency of monitoring when initiating or adjusting the dose of RAYALDEE [see Dosage and Administration (2)].
5.3 Adynamic Bone Disease
Adynamic bone disease with subsequent increased risk of fractures may develop if intact PTH levels are suppressed by RAYALDEE to abnormally low levels. Monitor intact PTH levels and adjust RAYALDEE dose, if needed [see Dosage and Administration (2.2)].
6 ADVERSE REACTIONS
The following important adverse reactions are discussed in greater detail in other sections of the label:
- •
- Hypercalcemia [see Warnings and Precautions (5.1)]
- •
- Adynamic Bone Disease [see Warnings and Precautions (5.3)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice.
The data in Table 1 are derived from two pivotal studies described below [see Clinical Studies (14)]. These data reflect exposure of 285 subjects to RAYALDEE 30 or 60 mcg daily for up to 6 months (mean 24 weeks, range 1 to 31 weeks). The mean age of the study population was 66 years old (range 25-85 years). Half of the subjects were male, 65% were White, and 32% were African-American or Black. At baseline, subjects had secondary hyperparathyroidism, stage 3 (52%) or 4 (48%) chronic kidney disease without macroalbuminuria and serum total 25-hydroxyvitamin D levels less than 30 ng/mL. The most common causes of chronic kidney disease were diabetes and hypertension and the mean estimated GFR at baseline was 31 mL/min/1.73m2. At baseline, mean plasma intact PTH was 148 pg/mL, mean serum calcium was 9.2 mg/dL, mean serum phosphorus was 3.7 mg/dL and mean serum 25-hydroxyvitamin D was 20 ng/mL.
Table 1 shows common adverse reactions associated with the use of RAYALDEE in the pooled placebo-controlled trials. These adverse reactions were not present at baseline, occurred more commonly on RAYALDEE than on placebo, and occurred in at least 1.4% of patients treated with RAYALDEE.
| Adverse Reaction | Placebo
N=144 | RAYALDEE
N=285 |
|---|---|---|
|
% |
% |
|
|
Anemia |
3.5 |
4.9 |
|
Nasopharyngitis |
2.8 |
4.9 |
|
Blood creatinine increased |
1.4 |
4.9 |
|
Dyspnea |
2.8 |
4.2 |
|
Cough |
2.1 |
3.5 |
|
Cardiac failure congestive |
0.7 |
3.5 |
|
Constipation |
2.8 |
3.2 |
|
Bronchitis |
0.7 |
2.8 |
|
Hyperkalemia |
0.7 |
2.5 |
|
Osteoarthritis |
0.7 |
2.1 |
|
Hyperuricemia |
0.7 |
1.8 |
|
Contusion |
0.0 |
1.8 |
|
Pneumonia |
0.7 |
1.4 |
|
Chronic obstructive pulmonary disease |
0.0 |
1.4 |
Increase in Serum Calcium
Patients randomized to RAYALDEE experienced a greater mean (SE) increase in serum calcium (P<0.001) than patients randomized to placebo [i.e., 0.2 (0.02) mg/dL on RAYALDEE versus 0.1 (0.03) mg/dL on placebo from baseline to trial end]. Six subjects (2%) in the RAYALDEE treatment group and no subjects (0%) in the placebo group required dose reductions for protocol-defined hypercalcemia (two consecutive serum calcium values greater than 10.3 mg/dL). A total of 4.2% of RAYALDEE treated subjects and 2.1% of placebo treated subjects experienced at least one elevation in serum calcium above the upper limit of normal (10.5 mg/dL).
Increase in Serum Phosphorus
Patients randomized to RAYALDEE experienced a greater mean (SE) increase in serum phosphorus than patients randomized to placebo [i.e., 0.2 (0.03) mg/dL on RAYALDEE versus 0.1 (0.04) mg/dL on placebo from baseline to trial end]. One subject (0.4%) in the RAYALDEE treatment group met protocol-defined hyperphosphatemia (two consecutive serum phosphorus values greater than 5.5 mg/dL deemed to be study drug related) compared to no subjects in the placebo group. A total of 45% of RAYALDEE treated subjects and 44% of placebo treated subjects experienced at least one elevation in serum phosphorus above the upper limit of normal (4.5 mg/dL).
7 DRUG INTERACTIONS
7.1 CYP3A Inhibitors
Cytochrome P450 inhibitors, such as ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin or voriconazole, may inhibit enzymes involved in vitamin D metabolism (CYP24A1 and CYP27B1), and may alter serum levels of calcifediol. Dose adjustment of RAYALDEE may be required, and serum 25-hydroxyvitamin D, intact PTH and serum calcium concentrations should be closely monitored if a patient initiates or discontinues therapy with a strong CYP3A4 inhibitor.
7.2 Thiazides
Thiazides are known to induce hypercalcemia by reducing excretion of calcium in the urine.
Concomitant administration of thiazides with RAYALDEE may cause hypercalcemia. Patients may require more frequent serum calcium monitoring in this setting [see Warnings and Precautions (5.1)].
7.3 Cholestyramine
Cholestyramine has been reported to reduce intestinal absorption of fat-soluble vitamins and may impair the absorption of calcifediol, the active ingredient in RAYALDEE. Dose adjustment of RAYALDEE may be required, and serum total 25-hydroxyvitamin D, intact PTH and serum calcium concentrations should be closely monitored if a patient initiates or discontinues therapy with cholestyramine.
7.4 Other Agents
Phenobarbital or other anticonvulsants or other compounds that stimulate microsomal hydroxylation reduce the half-life of calcifediol, the active ingredient in RAYALDEE. Dose adjustment of RAYALDEE may be required, and serum total 25-hydroxyvitamin D, intact PTH and serum calcium concentrations should be closely monitored if a patient initiates or discontinues therapy with phenobarbital or other anticonvulsants.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no human data with calcifediol use in pregnant women to identify a drug-associated risk for major birth defects, miscarriage or adverse maternal or fetal outcomes. There are risks to the mother and fetus associated with chronic kidney disease in pregnancy (see Clinical Considerations). In animal reproduction studies, calcifediol increased skeletal and soft tissue malformation when rabbits were given once daily oral doses representing ≥8 times the human dose of 60 mcg/day, based on body surface area (mg/m2), during the period of organogenesis. No adverse developmental effects were observed in rats given up to 10 times the human dose, based on body surface area (mg/m2), during organogenesis.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Chronic kidney disease in pregnancy increases the maternal risk for hypertension, miscarriage, preterm labor, and preeclampsia. Chronic kidney disease increases the fetal risk for intrauterine growth restriction (IUGR), prematurity, polyhydramnios, still birth, and low birth weight.
Data
Animal Data
Calcifediol was given orally to pregnant rats and rabbits during the period of organogenesis (in rats, from gestation day [GD] 6 to 15; in rabbits, from GD 6 to 18). Rats were given 0, 12, 40, 60 mcg/kg/day; and rabbits were given 0, 5, 25, 50 mcg/kg/day, representing up to 10 and 16 times, respectively, a human dose of 60 mcg/day, based on body surface area (mg/m2).
In rats, no adverse developmental effects were observed at calcifediol doses up to 60 mcg/kg/day. In rabbits, increased incidences of domed skull, enlarged atrium of the heart, and dilatation of pulmonary artery, were observed at doses of 25 and 50 mcg/kg/day (representing 8 and 16 times the human dose of 60 mcg/day, respectively, based on body surface area (mg/m2). Rats were given calcifediol during the pre/postnatal period (GD 15 to weaning). No adverse effects on gestation, parturition, lactation or survival of offspring were observed at calcifediol doses up to 60 mcg/kg/day.
8.2 Lactation
Risk Summary
There is no information available on the presence of calcifediol in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. Infants potentially exposed to calcifediol through breast milk should be monitored for signs and symptoms of hypercalcemia (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RAYALDEE and any potential adverse effects on the breastfed child from RAYALDEE or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of RAYALDEE have not been established in pediatric patients.
8.5 Geriatric Use
Of the total number of subjects in phase 3 placebo-controlled clinical studies of RAYALDEE, 63% were ≥65 years of age and 22% were ≥75 years of age. No overall differences in the safety or efficacy of RAYALDEE were observed between subjects older than 65 years and younger subjects.
8.6 Renal Impairment
No difference in efficacy was observed between patients with stage 3 chronic kidney disease or those with stage 4 disease in subgroup analysis. Safety outcomes were similar in these subgroups. The safety and efficacy of RAYALDEE in the treatment of secondary hyperparathyroidism in patients with stage 2 or stage 5 chronic kidney disease and patients with end-stage renal disease on dialysis have not been established [see Indications and Usage (1)].
10 OVERDOSAGE
Excessive administration of RAYALDEE can cause hypercalciuria, hypercalcemia, hyperphosphatemia, or oversuppression of intact PTH. Common symptoms of vitamin D overdosage may include constipation, decreased appetite, dehydration, fatigue, irritability, muscle weakness, or vomiting.
Treatment of acute accidental overdosage with RAYALDEE should consist of general supportive measures. If the overdosage is discovered within a short time, induce emesis or perform gastric lavage to prevent further absorption. Obtain serial serum and urine calcium measurements, and assess any electrocardiographic abnormalities due to hypercalcemia. Discontinue supplemental calcium. Treat with standard medical care if persistent and markedly elevated serum calcium levels occur.
Calcifediol is not significantly removed by dialysis.
11 DESCRIPTION
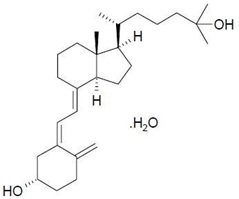
Calcifediol, USP, the active ingredient in RAYALDEE, is synthetically manufactured as calcifediol monohydrate. Calcifediol is also known as calcidiol, 25-hydroxycholecalciferol or 25-hydroxyvitamin D3.
Calcifediol monohydrate is a white crystalline powder, has a calculated molecular weight of 418.65 and is soluble in alcohol and fatty oils but practically insoluble in water. Chemically, calcifediol monohydrate is (3β,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-3,25-diol monohydrate and its structural formula is:
RAYALDEE is formulated as extended-release capsules containing 30 mcg of calcifediol.
RAYALDEE is available as soft capsules or two-piece banded hard capsules containing 30 mcg of calcifediol for oral administration. Each capsule contains the following excipients: butylated hydroxytoluene, dehydrated alcohol, hypromellose, lauroyl polyoxylglycerides, mineral oil, monoglycerides and diglycerides, and paraffin. The soft capsule shells contain carrageenan, FD&C Blue #1, modified starch, purified water, sodium phosphate dibasic, sorbitol sorbitan solution, and titanium dioxide. Medium chain triglyceride (fractionated coconut) oil is used as a lubricant during manufacture, and trace amounts may be present in the final formulation. The hard capsule shells contain gellan gum, hypromellose, and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Calcifediol (25-hydroxyvitamin D3) is a prohormone of the active form of vitamin D3, calcitriol (1,25‑dihydroxyvitamin D3). Calcifediol is converted to calcitriol by cytochrome P450 27B1 (CYP27B1), also called 1-alpha hydroxylase, primarily in the kidney. Calcitriol binds to the vitamin D receptor in target tissues and activates vitamin D responsive pathways that result in increased intestinal absorption of calcium and phosphorus and reduced parathyroid hormone synthesis.
12.2 Pharmacodynamics
In repeat-dose clinical studies with RAYALDEE, increased levels of serum total 25-hydroxyvitamin D were associated with corresponding increases in serum total 1,25‑dihydroxyvitamin D concentrations and reductions in circulating plasma intact PTH observed within the first two weeks of RAYALDEE treatment [see Clinical Studies (14)].
12.3 Pharmacokinetics
Absorption
Exposure to calcifediol increased proportionally over the dose range of 30 to 90 mcg following repeated daily administration of RAYALDEE at bedtime to subjects with secondary hyperparathyroidism, chronic kidney disease and vitamin D insufficiency. Steady-state levels of serum total 25-hydroxyvitamin D were reached after approximately 3 months [see Clinical Studies (14)].
Effect of Food
Food effect studies with a supratherapeutic dose of RAYALDEE at 450 mcg or 900 mcg in healthy subjects resulted in an approximately 5-fold increase in maximum serum calcifediol concentration (Cmax) and a 3.5-fold increase in AUC0-t when administered with a high fat, high calorie meal compared to fasting.
Distribution
Calcifediol is extensively bound to plasma proteins (>98%). The mean apparent volume of distribution is 8.8 L in healthy subjects following a single oral dose of RAYALDEE, and 30.1 L in subjects with stage 3 or 4 chronic kidney disease following repeated dosing.
Elimination
The mean elimination half-life of calcifediol is approximately 11 days in healthy individuals following a single dose of RAYALDEE, and approximately 25 days in patients with stage 3 or stage 4 chronic kidney disease following repeated once daily dosing.
Metabolism
Production of calcitriol from calcifediol is catalyzed by the 1-alpha-hydroxylase enzyme, CYP27B1, located in the kidney and other tissues. CYP24A1, located in all vitamin D-responsive tissues, catabolizes both calcifediol and calcitriol to inactive metabolites.
Excretion
Excretion of calcifediol occurs primarily through the biliary fecal route.
Specific Populations
Age, gender and race had no meaningful impact on steady-state concentrations of calcifediol following RAYALDEE administration.
Patients with Renal Impairment
There was no meaningful difference in calcifediol steady-state concentrations following repeated RAYALDEE administration in patients with stage 3 or stage 4 chronic kidney disease.
Patients with Hepatic Impairment
The pharmacokinetics of calcifediol have not been investigated in patients with hepatic impairment.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No neoplastic changes attributable to calcifediol were observed at subcutaneous doses of 3, 10 and 33 mcg/kg/day in a 26-week rasH2 transgenic mouse study.
In vitro or in vivo mutagenicity studies have not been performed with RAYALDEE.
Calcifediol has not been shown to have significant effects on fertility in rats.
14 CLINICAL STUDIES
The efficacy and safety of RAYALDEE were evaluated in two identical multicenter, randomized, placebo-controlled, double-blind trials in patients with secondary hyperparathyroidism, stage 3 or 4 chronic kidney disease and serum total 25-hydroxyvitamin D levels between 10 and 30 ng/mL. Subjects were stratified by chronic kidney disease stage and randomized in a 2:1 ratio to receive RAYALDEE or a matching placebo at bedtime over 26 weeks. The dose of RAYALDEE was 30 mcg once daily for the first 12 weeks and either 30 or 60 mcg once daily for the last 14 weeks. The dose was increased to 60 mcg at the start of week 13 if the plasma intact PTH level was greater than 70 pg/mL, the serum 25-hydroxyvitamin D level was less than 65 ng/mL and the serum calcium level was less than 9.8 mg/dL.
A total of 213 subjects were randomized in one trial (72 received placebo and 141 received RAYALDEE), and 216 subjects were randomized in the second trial (72 received placebo and 144 received RAYALDEE). The subjects’ mean age was 66 years (range 25-85), 50% were male, 65% White, 32% African-American or Black and 3% Other. At baseline, subjects had secondary hyperparathyroidism, and stage 3 (52%) or stage 4 (48%) chronic kidney disease without macroalbuminuria. The most common causes of chronic kidney disease were diabetes and hypertension and the mean estimated GFR was 31 mL/min/1.73m2. Mean baseline intact PTH was 130 pg/mL for subjects with stage 3 disease (n=222) and 166 pg/mL for subjects with stage 4 disease (n=207). Mean serum calcium was 9.2 mg/dL, mean serum phosphorus was 3.7 mg/dL and mean serum 25-hydroxyvitamin D was 20 ng/mL. Of the 429 subjects randomized, 354 (83%) completed the studies.
The primary analysis compared the proportion of individuals who experienced an at least 30% reduction in plasma intact PTH from baseline to end of trial (average of weeks 20, 22, 24 and 26). A larger proportion of patients randomized to RAYALDEE experienced an at least 30% reduction in plasma intact PTH from baseline compared to placebo in both trials [33% versus 8% in the first trial (P<0.001) and 34% versus 7% in the second trial (P<0.001)].
A description of mean (SE) percent change in plasma intact PTH from baseline across study visits in the two trials combined is shown in Figure 1. Serum total 25-hydroxyvitamin D levels increased to at least 30 ng/mL in 80% and 83% of subjects treated with RAYALDEE vs. 3% and 7% of subjects treated with placebo (P<0.001) in the two studies, respectively. Average steady-state 25-hydroxyvitamin D levels were 50 and 56 ng/mL for subjects receiving 30 mcg daily, and 69 and 67 ng/mL for subjects receiving 60 mcg daily, in the first and second studies, respectively.
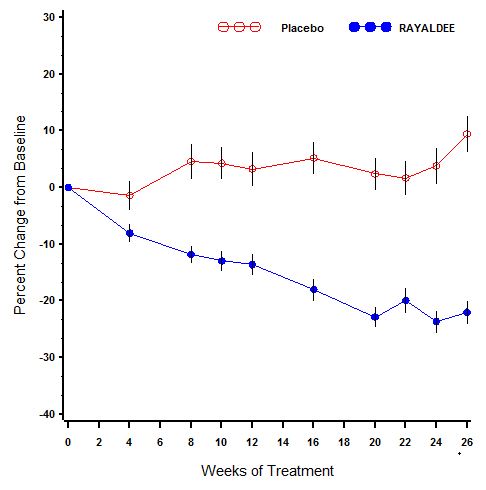
Figure 1. Mean (±SE) Percent Change from Baseline in Plasma Intact PTH in the Per Protocol Populations (Pooled Data from Two Phase 3 Studies)
The Per Protocol (PP) population consisted of all subjects with at least 2 intact PTH values in the calculated baseline and efficacy assessment period (EAP) values and who did not have a major protocol deviation during the treatment period of the study. The PP population comprised 83% of randomized subjects.
16 HOW SUPPLIED/STORAGE AND HANDLING
RAYALDEE is supplied as 30 mcg calcifediol in blue, oval extended-release soft capsules, imprinted O:
Bottles of 30 [NDC 70301-1001-1]
Bottles of 60 [NDC 70301-1001-2]
RAYALDEE is also supplied as 30 mcg calcifediol in white extended-release two-piece banded hard capsules, imprinted O on the cap and 30 on the body:
Bottles of 30 [NDC 70301-1002-1]
Bottles of 60 [NDC 70301-1002-2]
Store at 20 to 25°C (68 to 77°F); excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
- •
- Inform patients to take RAYALDEE at bedtime and to swallow the capsules whole.
- •
- Inform patients if they miss a dose, to take RAYALDEE at the next scheduled time. Do not take an extra dose to make up for the missed dose.
- •
- Inform patients that they will need routine monitoring of laboratory parameters such as calcium, iPTH and total 25-hydroxyvitamin D while taking RAYALDEE.
- •
- Advise patients to contact a health care provider if they develop symptoms of elevated calcium (e.g., feeling tired, having difficulty thinking clearly, loss of appetite, nausea, vomiting, constipation, increased thirst, increased urination or weight loss).
- •
- Advise patients to inform their physician of all use of medications, including prescription and nonprescription drugs, supplements and herbal preparations, and of any changes in medical condition. Patients should also be advised to inform their physicians, when receiving a newly prescribed medication, that they are taking RAYALDEE.
- •
- Inform lactating women about the need to monitor infants exposed to RAYALDEE through breast milk for signs of hypercalcemia to include seizures, vomiting, constipation and weight loss [see Use in Specific Populations (8.2)].
RAYALDEE® is a registered trademark of Eirgen Pharma Ltd.
Patent: https://www.opko.com/what-we-do/our-research/patents
Manufactured for:
OPKO Pharmaceuticals, LLC
4400 Biscayne Blvd.
Miami FL 33137 USA
© 2024 OPKO Pharmaceuticals, LLC. All rights reserved.
PRINCIPAL DISPLAY PANEL NDC: 70301-1001-1 - 30-count Bottle Label
NDC 70301-1001-1 OPKO
Rayaldee® (calcifediol)
Extended-Release Capsules
30 mcg
Rx only
Each capsule contains:
30 mcg calcifediol
See package insert for full
prescribing information
30 Capsules
Manufactured for:
OPKO Pharmaceuticals, LLC
4400 Biscayne Blvd
Miami FL 33137
Rev. 03/21
PC2192
Store between 20 - 25°C
(68-77°F) excursions
permitted to 15 - 30°C
(59 - 86°F) see [USP
controlled room temperature]
PRINCIPAL DISPLAY PANEL NDC: 70301-1002-1 - 30-count Bottle Label
NDC 70301-1002-1 OPKO
Rayaldee® (calcifediol)
Extended-Release Capsules
30 mcg
Rx only
Each capsule contains:
30 mcg calcifediol
See package insert for full
prescribing information
30 Capsules
Manufactured for:
OPKO Pharmaceuticals, LLC
4400 Biscayne Blvd
Miami FL 33137
Rev. 04/22
PC2333
Store between 20 - 25°C
(68-77°F) excursions
permitted to 15 - 30°C
(59 - 86°F) see [USP
controlled room temperature]