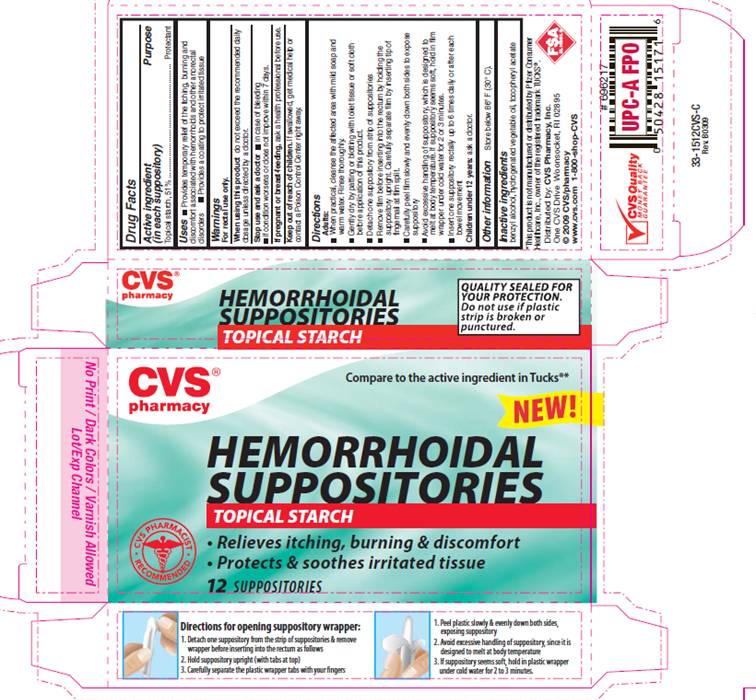
USE
- Provides temporary relief of the itching, burning and discomfort associated with hemorrhoids and other anorectal disorders
- Provides a coating to protect irritated tissue
WARNINGS
For rectal use only.
DIRECTIONS
Adults:- When practical, cleanse the affected area with mild soap and warm water. Rinse thoroughly.
- Gently dry by patting or blotting with toilet tissue or soft cloth before application of this product.
- Detach one suppository from strip of suppositories.
- Remove film before inserting into the rectum by holding the suppository upright. Carefully separate film by inserting tip of fingernail at film split.
- Carefully peel film slowly and evenly down both sides to expose suppository
- Avoid excessive handling of suppository, which is designed to melt at body temperature. If suppository seems soft, hold in film wrapper under cold water for 2 or 3 minutes.
- Insert one suppository rectally up to 6 times daily or after each bowel movement
PACKAGE INFORMATION - REPRESENTATIVE LABEL
Compare to the active ingredient in TUCKS®*HEMORRHOIDAL
SUPPOSITORIES
TOPICAL STARCH
- Relieves itching, burning and discomfort
- Protects and soothes irritated tissue
*This product is not manufactured or distributed by Pfizer consumer Healthcare, Inc., owner of the registered trademark TUCKS®.