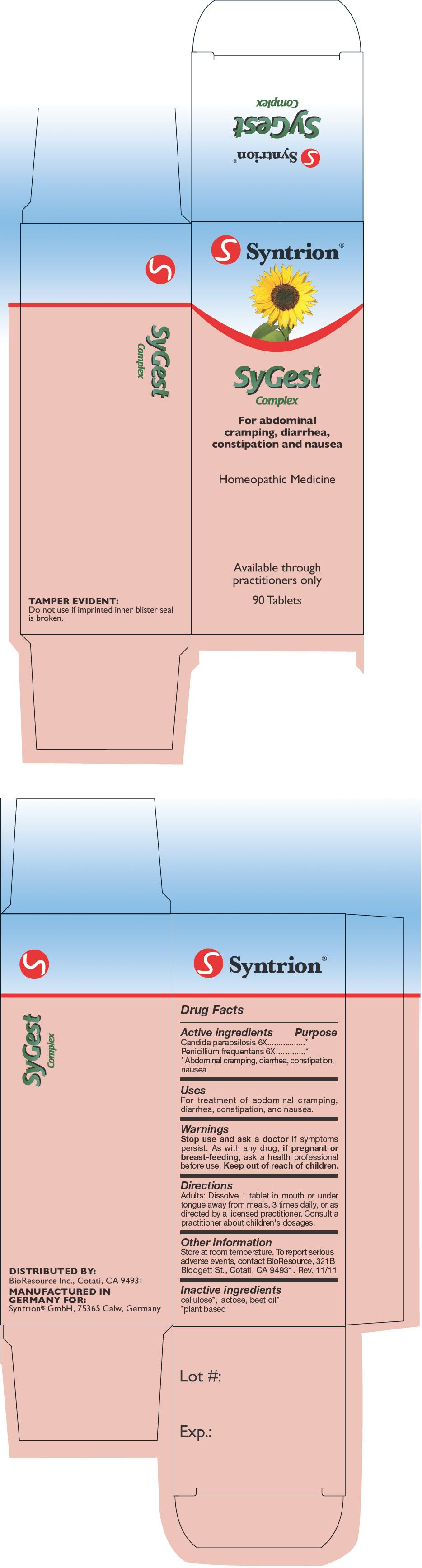
SYGEST COMPLEX- candida parapsilosis and penicillium glabrum tablet
Syntrion GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
SyGest Complex 6X
| SYGEST COMPLEX
candida parapsilosis and penicillium glabrum tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Syntrion GmbH (331883145) |
Revised: 1/2015
Document Id: bf8cc2d9-6c98-4386-8ee6-040e7ea9f4ac
Set id: 69f680c3-2bd0-4a5d-82bb-320695e9eb5f
Version: 2
Effective Time: 20150109
Syntrion GmbH