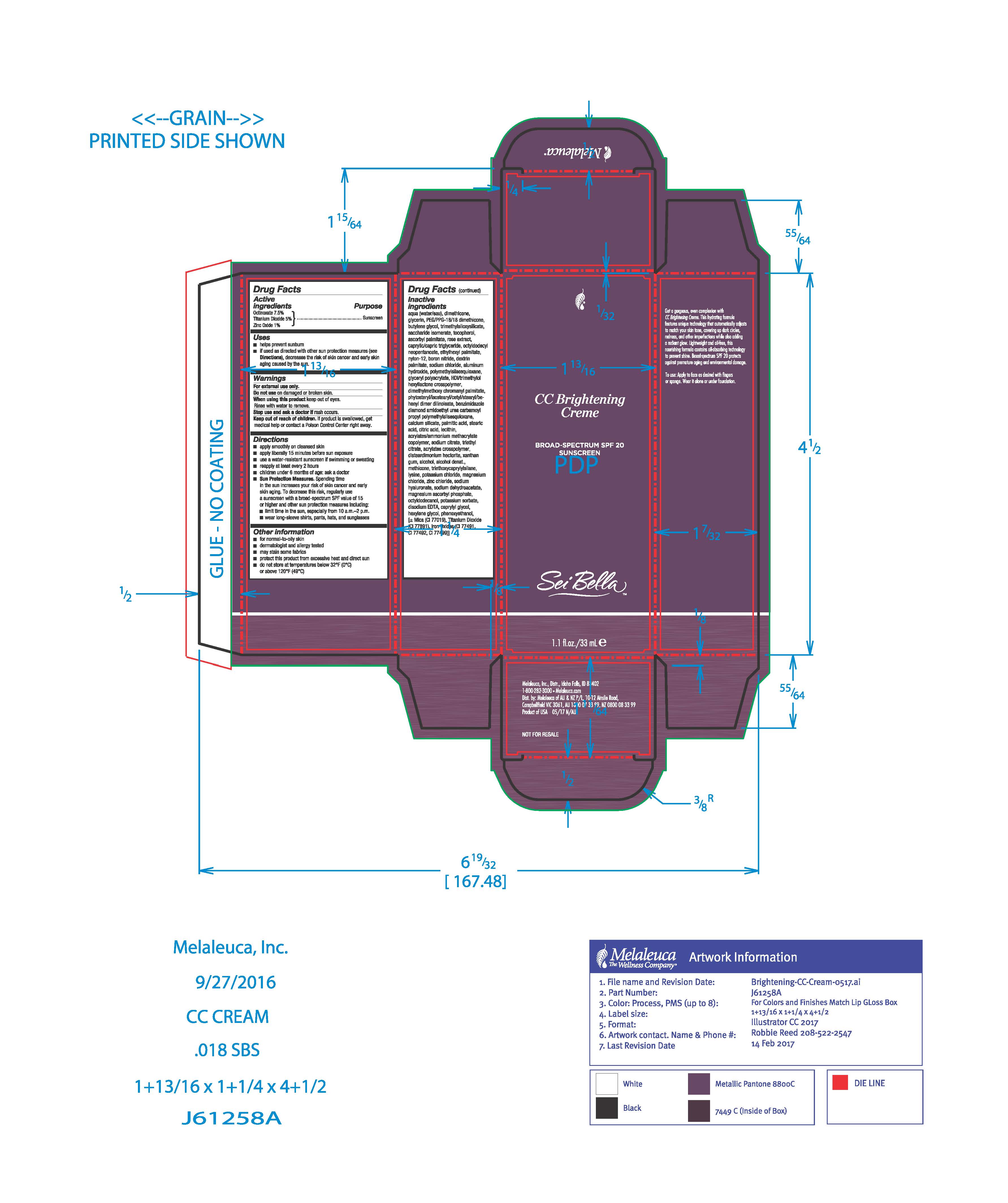
Use
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), descreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
■ apply after cleanser, toner, and foundation primer. Blend outward with Sei Bella foundation brush or fingers.
■ apply liberally 15 minutes before sun exposure
■ use a water-resistant sunscreen if swimming or sweating
■ reapply at least every 2 hours
■ children under 6 months: ask a doctor
■ Sun Protection Measures. Spending time in the sun increases you risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF value of 15 or higher and other sun protection measures including:
■ limit time in the sun, especially from 10 a.m.-2 p.m.
■ wear long-sleeve shirts, pants, hats, and sunglasses
Inactive ingredients
Aqua/Water/Eau, Dimethicone, Glycerin, PEG/PPG-18/18 Dimethicone, Butylene Glycol, Trimethylsiloxysilicate, Saccharide Isomerate, Tocopherol, Ascorbyl Palmitate, Rose Extract, Caprylic/Capric Triglyceride, Octyldodecyl Neopentanoate , Ethylhexyl Palmitate, Nylon-12, Boron Nitride, Dextrin Palmitate, Sodium Chloride, Aluminum Hydroxide, Polymethylsilsesquioxane, Glyceryl Polyacrylate, HDI/Trimethylol Hexyllactone Crosspolymer, Dimethylmethoxy Chromanyl Palmitate, Phytosteryl/Isostearyl/Cetyl/Stearyl/Behenyl Dimer Dilinoleate, Benzimidazole Diamond Amidoethyl Urea Carbamoyl Propyl Polymethylsilsesquioxane, Calcium Silicate, Palmitic Acid, Stearic Acid, Citric Acid , Lecithin, Acrylates/Ammonium Methacrylate Copolymer, Sodium Citrate, Triethyl Citrate , Acrylates Crosspolymer, Disteardimonium Hectorite, Xanthan Gum, Alcohol, Alcohol Denat., Methicone, Triethoxycaprylylsilane, Lysine, Potassium Chloride, Magnesium Chloride, Zinc Chloride, Sodium Hyaluronate, Sodium Dehydroacetate, Magnesium Ascorbyl Phosphate, Octyldodecanol, Potassium Sorbate, Disodium EDTA, Caprylyl Glycol, Hexylene Glycol, Phenoxyethanol
May Contain (+/-)
Mica, Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499)