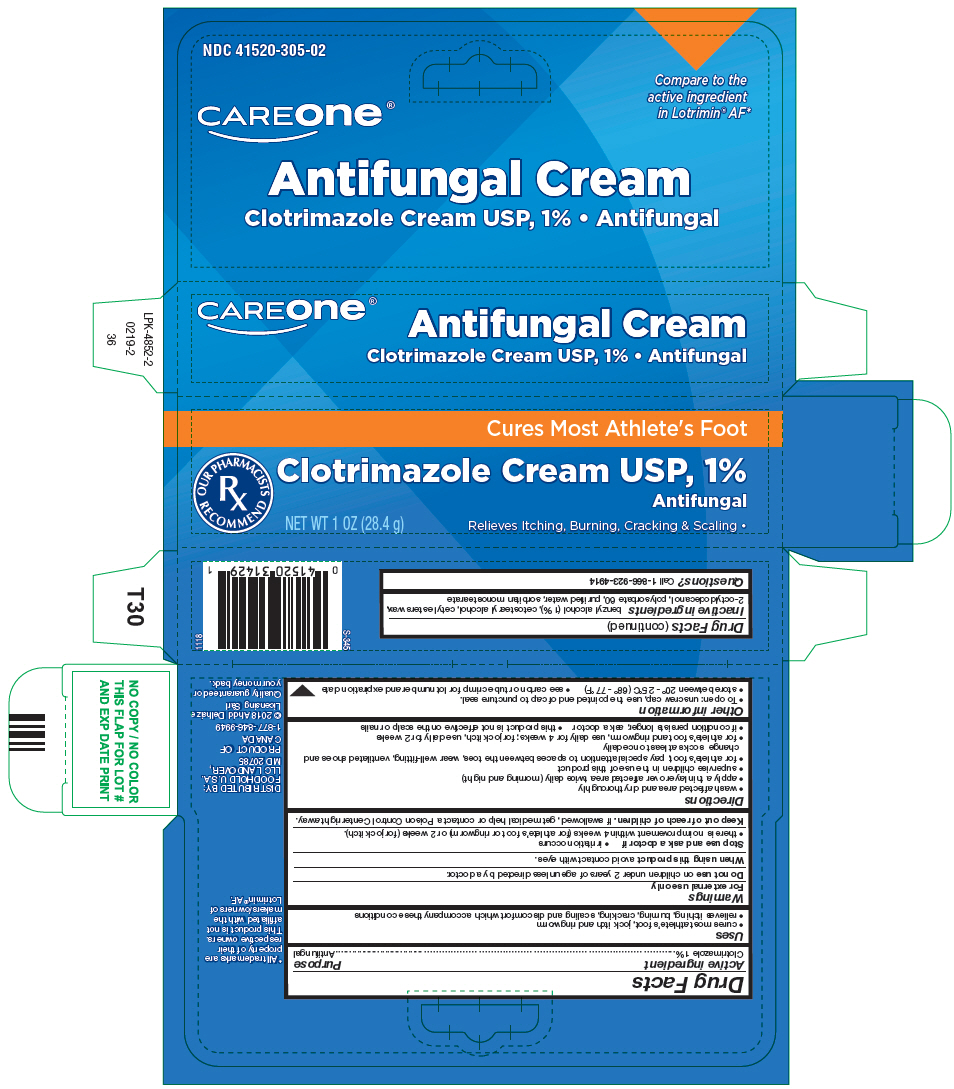
Uses
- cures most athlete's foot, jock itch and ringworm
- relieves itching, burning, cracking, scaling and discomfort which accompany these conditions
Warnings
For external use only
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- for athlete's foot, pay special attention to spaces between the toes, wear well-fitting, ventilated shoes and change socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- if condition persists longer, ask a doctor
- this product is not effective on the scalp or nails
Other information
- To open: unscrew cap, use the pointed end of cap to puncture seal.
- store between 20° - 25°C (68° - 77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
benzyl alcohol (1%), cetostearyl alcohol, cetyl esters wax, 2-octyldodecanol, polysorbate 60, purified water, sorbitan monostearate
PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
NDC 41520-305-02
CAREone®
Compare to the
active ingredient
in Lotrimin® AF*
Antifungal Cream
Clotrimazole Cream USP, 1% • Antifungal
Cures Most Athlete's Foot
OUR PHARMACISTS
RX
RECOMMEND
NET WT 1 OZ (28.4 g)
Relieves Itching, Burning, Cracking & Scaling •