Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
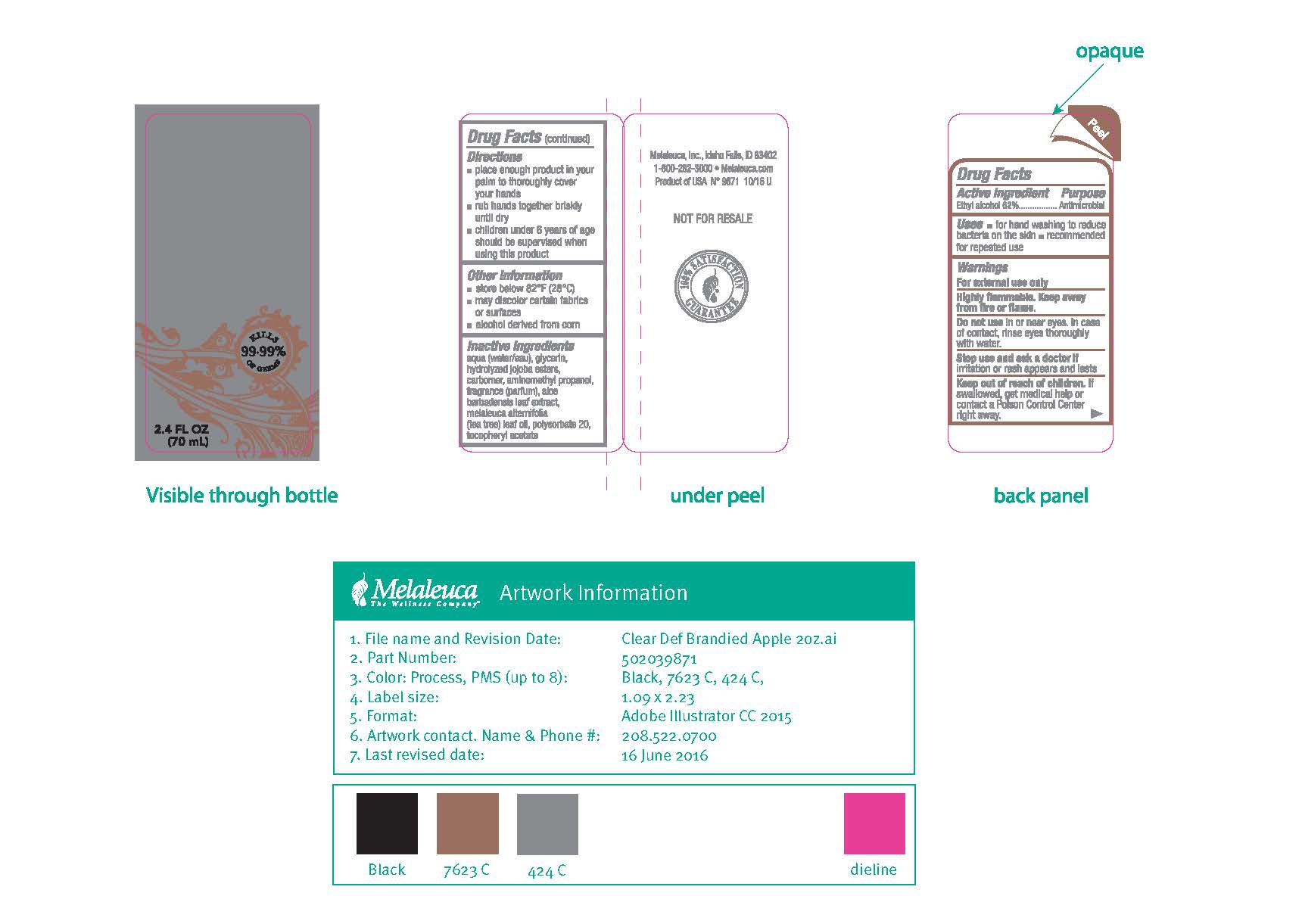
Directions ■ place enough product in your palm to thoroughly cover your hands ■ rub hands together briskly until dry ■ children under 6 years of age should be supervised when using this product
Other information ■ store below 82°F (28°C) ■ may discolor certain fabrics or surfaces ■ alcohol derived from corn
Inactive ingredients water, gylcerin, xylitol, anhydroxylitol, xylitylglucoside, carbomer, aminomethyl propanol, fragrance, aloe barbadensis leaf extract, melaleuca alternifolia leaf oil, polysorbate 20, tocopheryl acetate, jojoba esters, butyrospermum parkii, theobroma cacao seed butter, mangifera indica seed butter, blue 1 lake, titanium dioxide, yellow 5 lake