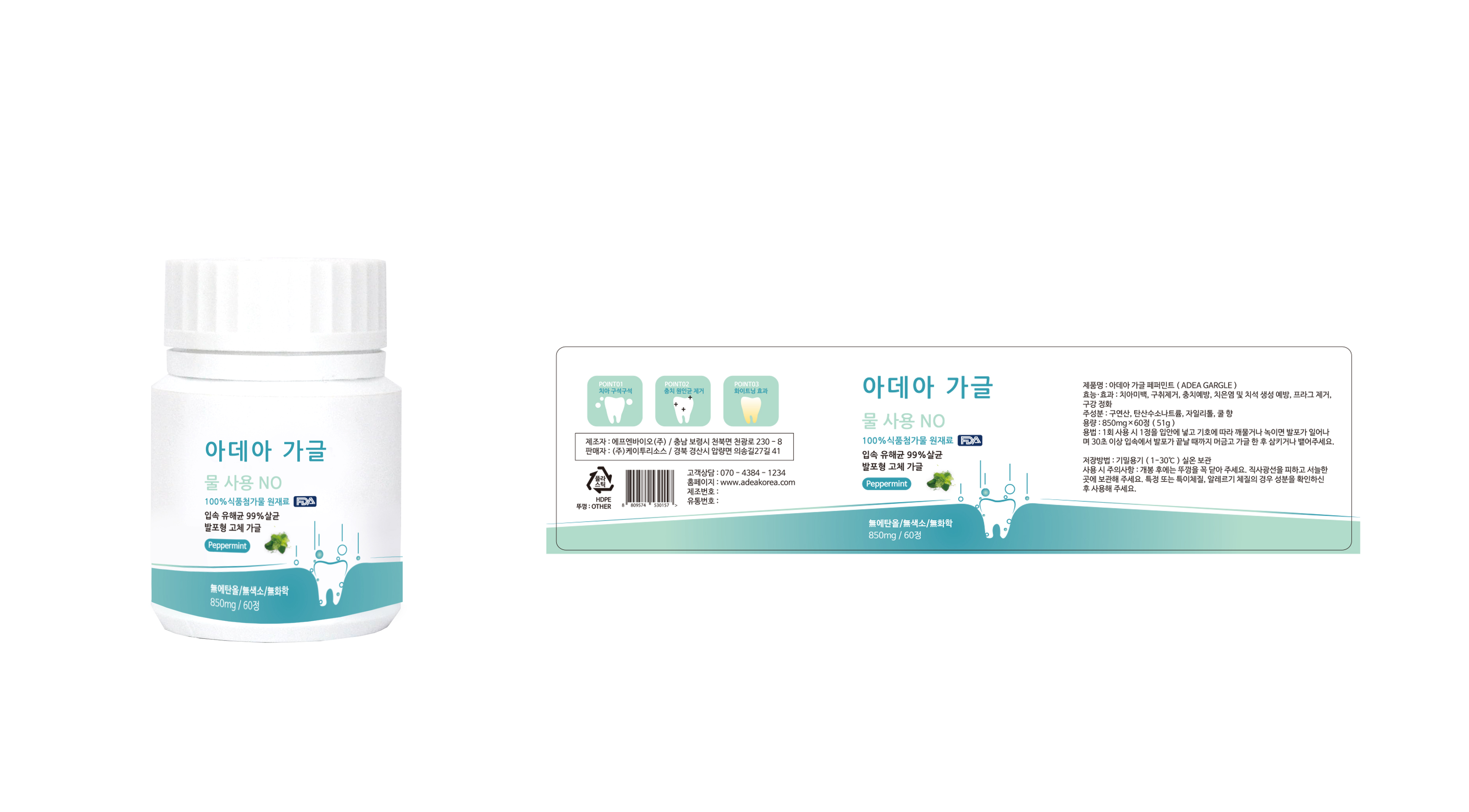
For oral care: Tooth whitening, removing bad breath, preventing tooth decay, preventing gingivitis and tartar formation, plaque removal, oral cleansing
Put a tablet of Adea Gargle in your mouth and chew or melt according to your preference.
When foaming starts, please gaggle for more than 30 seconds so that every corner of the mouth can reach Adea Gaggle.
When the gaggle is over, spit or swallow the bubbles.