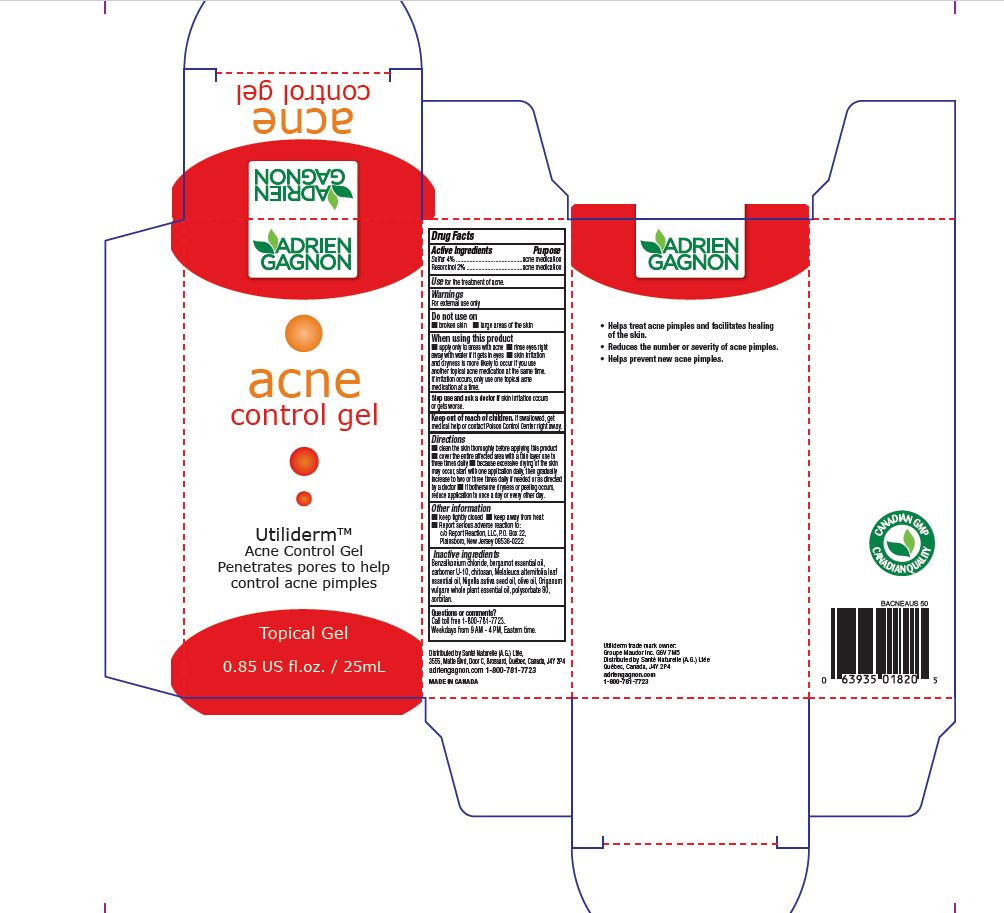
When using this product
■ apply only to areas with acne ■ rinse eyes right
away with water if it gets in eyes ■ skin irritation
and dryness is more likely to occur if you use
another topical acne medication at the same time.
If irritation occurs, only use one topical acne
medication at a time.
Keep out of reach of children. If swallowed, get
medical help or contact Poison Control Center right away.
Directions
■ clean the skin thoroughly before applying this product
■ cover the entire affected area with a thin layer one to
three times daily ■ because excessive drying of the skin
may occur, start with one application daily, then gradually
increase to two or three times daily if needed or as directed
by a doctor ■ if bothersome dryness or peeling occurs,
reduce application to once a day or every other day.
Other information
■ keep tightly closed ■ keep away from heat
■ Report serious adverse reaction to:
c/o Report Reaction, LLC, P.O. Box 22,
Plainsboro, New Jersey 08536-0222