Uses
- cures most jock itch
- relieves itching, burning, scaling, chafing and discomfort associated with jock itch
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- use daily for 2 weeks
- if condition persists longer, ask a doctor
- this product is not effective on the scalp or nails
Other information
- TAMPER EVIDENT: DO NOT USE IF THE SEAL ON THE TUBE IS PUNCTURED OR NOT VISIBLE.
- to open: unscrew cap, use the pointed end of cap to puncture seal.
- store between 20° to 25°C (68° to 77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
benzyl alcohol (1%), cetostearyl alcohol, cetyl esters wax, 2-octyldodecanol, polysorbate 60, purified water, sorbitan monostearate
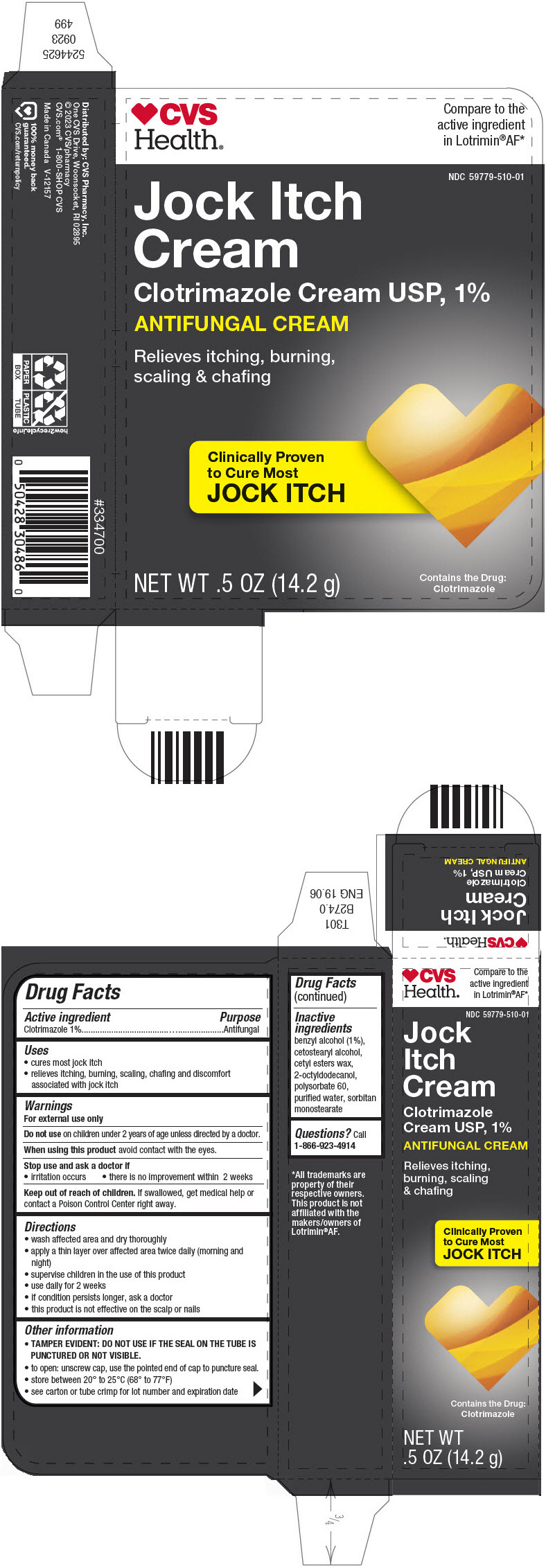
PRINCIPAL DISPLAY PANEL - 14.2 g Tube Carton
CVS
Health®
Compare to the
active ingredient
in Lotrimin®AF*
NDC 59779-510-01
Jock Itch
Cream
Clotrimazole Cream USP, 1%
ANTIFUNGAL CREAM
Relieves itching, burning,
scaling & chafing
Clinically Proven
to Cure Most
JOCK ITCH
NET WT .5 OZ (14.2 g)
Contains the Drug:
Clotrimazole