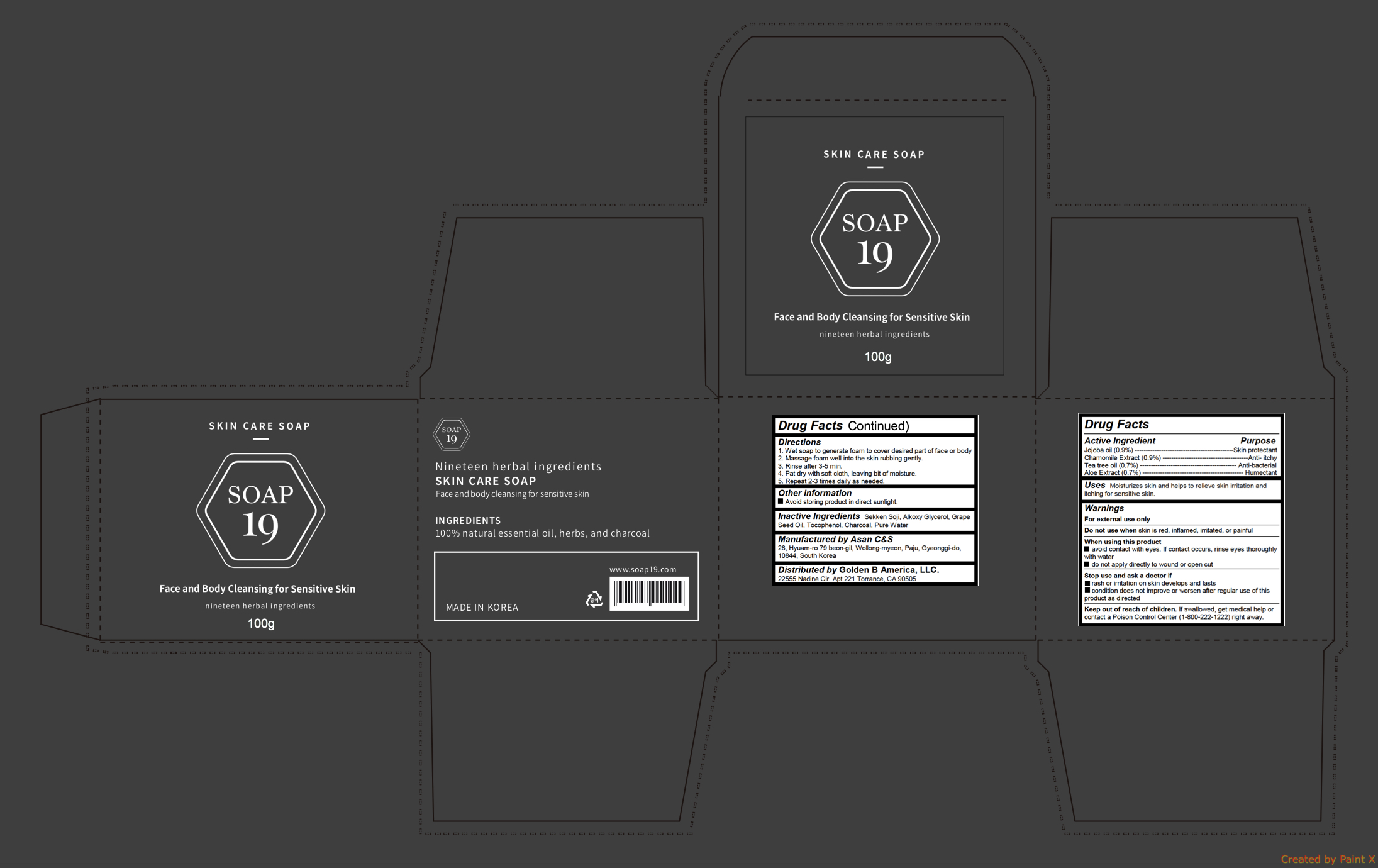
Active ingredients
Jojoba oil (0.9%)
Chamomile Extract (0.9%)
Tea tree oil (0.7%)
Aloe Extract (0.7%)
Warnings
For external use only
Do not use when skin is red, inflamed, irritated, or painful
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
- do not apply directly to wound or open cut
Stop use and ask a doctor if
- rash or irritation on skin develops and lasts
- condition does not improve or worsen after regular use of this product as directed
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.