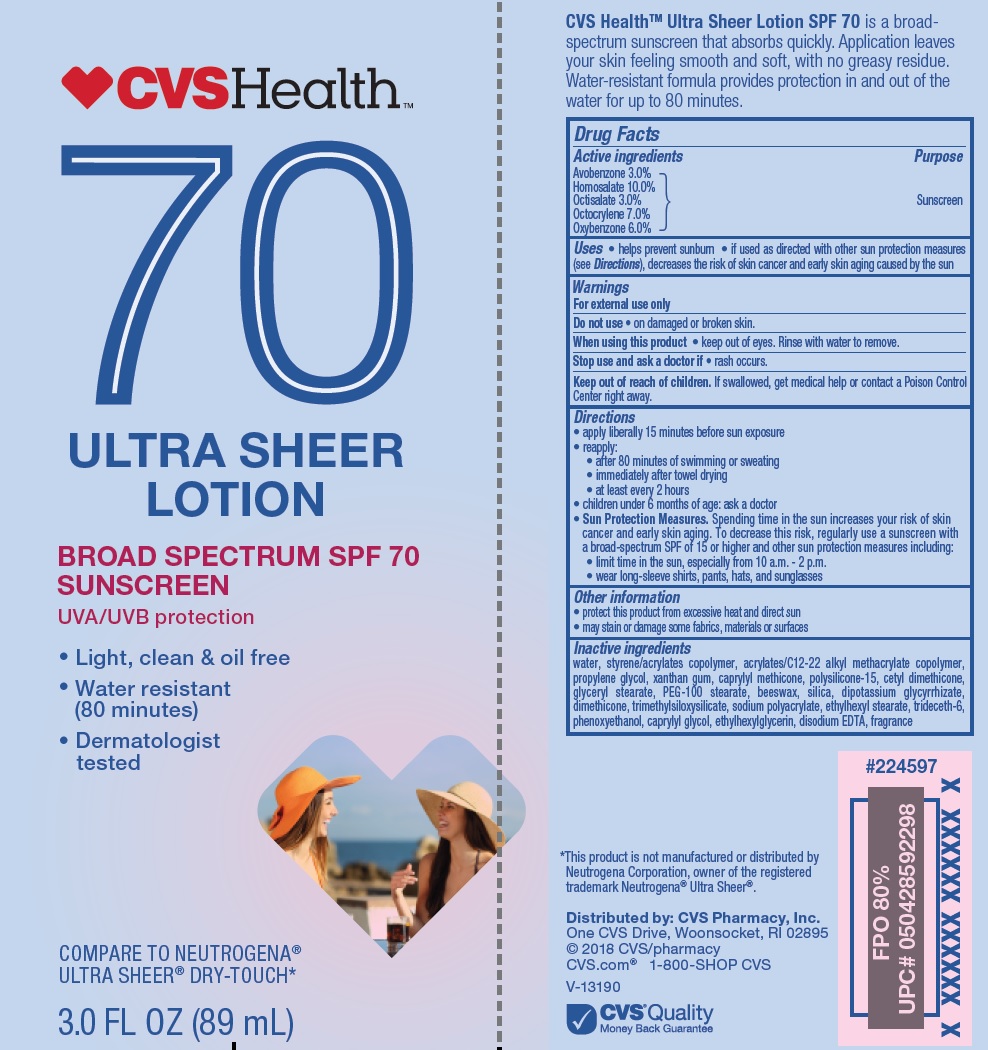
Active ingredients
Avobenzone 3.0%
Homosalate 10.0%
Octisalate 3.0%
Octocrylene 7.0%
Oxybenzone 6.0%
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
reapply:
• after 80 minutes of swimming or sweating
• immediately after towel drying
• at least every 2 hours
- children under 6 months of age: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. - 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
Other information
• protect the product in this container from excessive heat and direct sun
• may stain or damage some fabrics, materials or surfaces
Inactive ingredients
water, styrene/acrylates copolymer, dimethicone, acylates/C12-22 alkyl methacrylate copolymer, propylene glycol, xanthan gum, capryl methicone, polysilicone-15, cetyl dimethicone, glyceryl stearate, PEG-100 stearate, beeswax, silica, dipotassium glycyrrhizate, trimethylsiloxysilicate, sodium polyacrylate, ethylhexyl stearate, trideceth-6, phenoxyethanol, caprylyl glycol, ethylhexylglycerin, disodium EDTA, fragrance