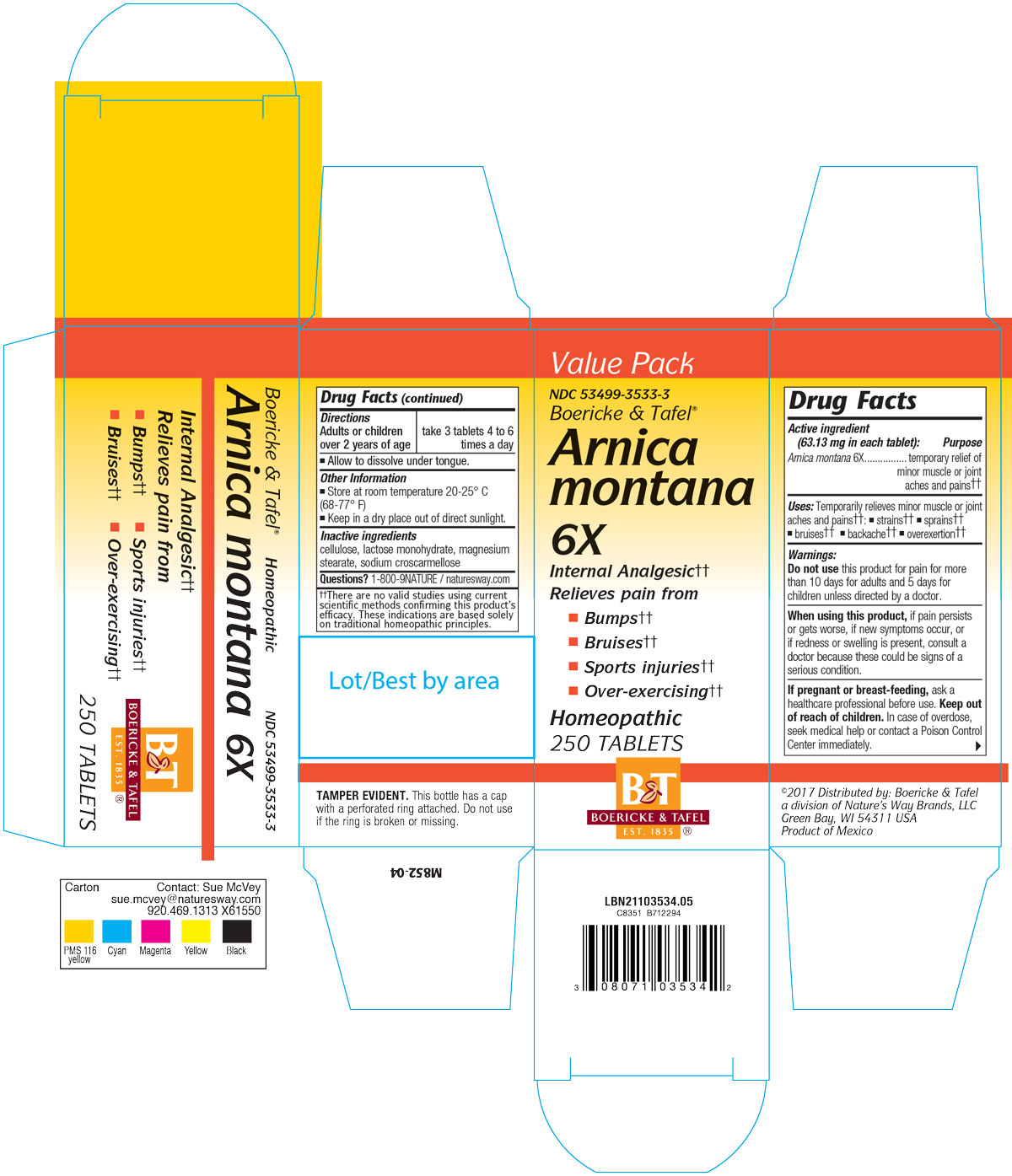
ARNICA MONTANA
- arnica montana tablet
Schwabe North America
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredient
Arnica montana 6X
Inactive Ingredient
CELLULOSE
LACTOSE MONOHYDRATE
MAGNESIUM STEARATE
SODIUM CROSCARMELLOSE
Dosage & Administration
Dosage:
Adults or children over 2 years: Take 3 tablets 4-6 times a day.
Allow to dissolve under tongue.
Indications
Temporarily relieves minor muscle or joint aches and pain; strains, sprains, bruises, backache or over-exertion.
Purpose
For the temporary relief of minor muscle or joint aches and pain; strains, sprains, bruises, backache or over-exertion.
Warning
Do not take this product for pain for more than 10 days (adult) or 5 days (children) unless directed by a doctor.
When Using
When using this product if pain persists or gets worse, if new symptoms occur, or if redness or swelling is present, consult a doctor because these could be signs of a serious condition.
Pregnancy and Breast Feeding
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Keep out of reach of children.
Overdosage
In case of overdose, seek medical help or contact a Poison Control Center immediately.