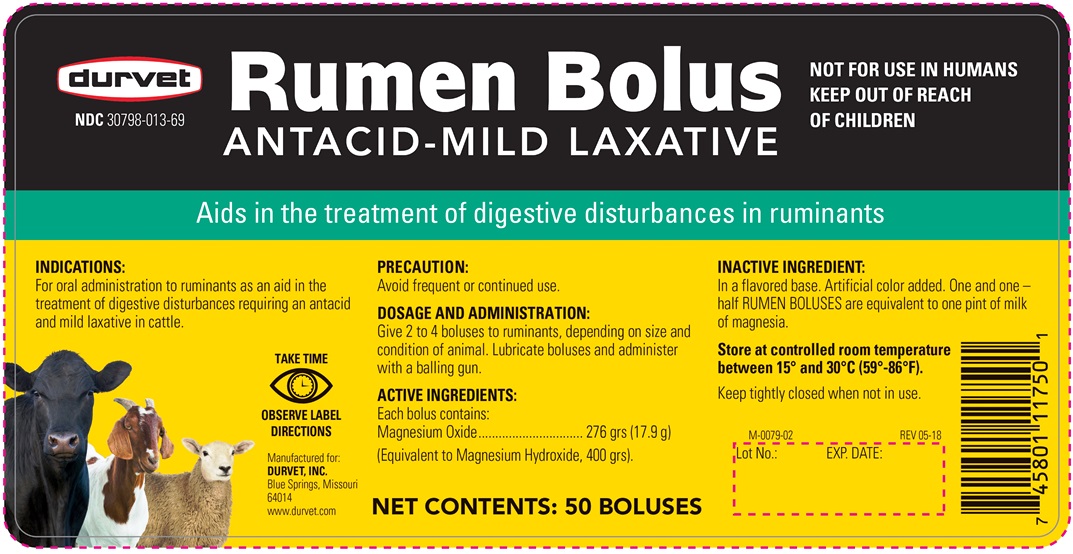
Antacid-Mild Laxative
Aids in the treatment of digestive disturbances in ruminants
FOR USE IN ANIMALS ONLY
KEEP OUT OF REACH OF CHILDREN
Indications:
For oral administration to ruminants as an aid in the treatment of digestive disturbances requiring an antacid and mild laxative in cattle.
Dosage And Administration
Give 2 to 4 boluses to ruminants, depending on size and condition of animal. Lubricate boluses and administer with a balling gun.
ACTIVE INGREDIENTS:
Each bolus contains:
Magnesium Oxide ... 276 gr (17.9 g)
(Equivalent to Magnesium Hydroxide, 400 grs.)
INACTIVE INGREDIENTS:
In a flavored base. Artificial color added One and one-half RUMEN BOLUSES are equivalent to one pint of milk of magnesia.