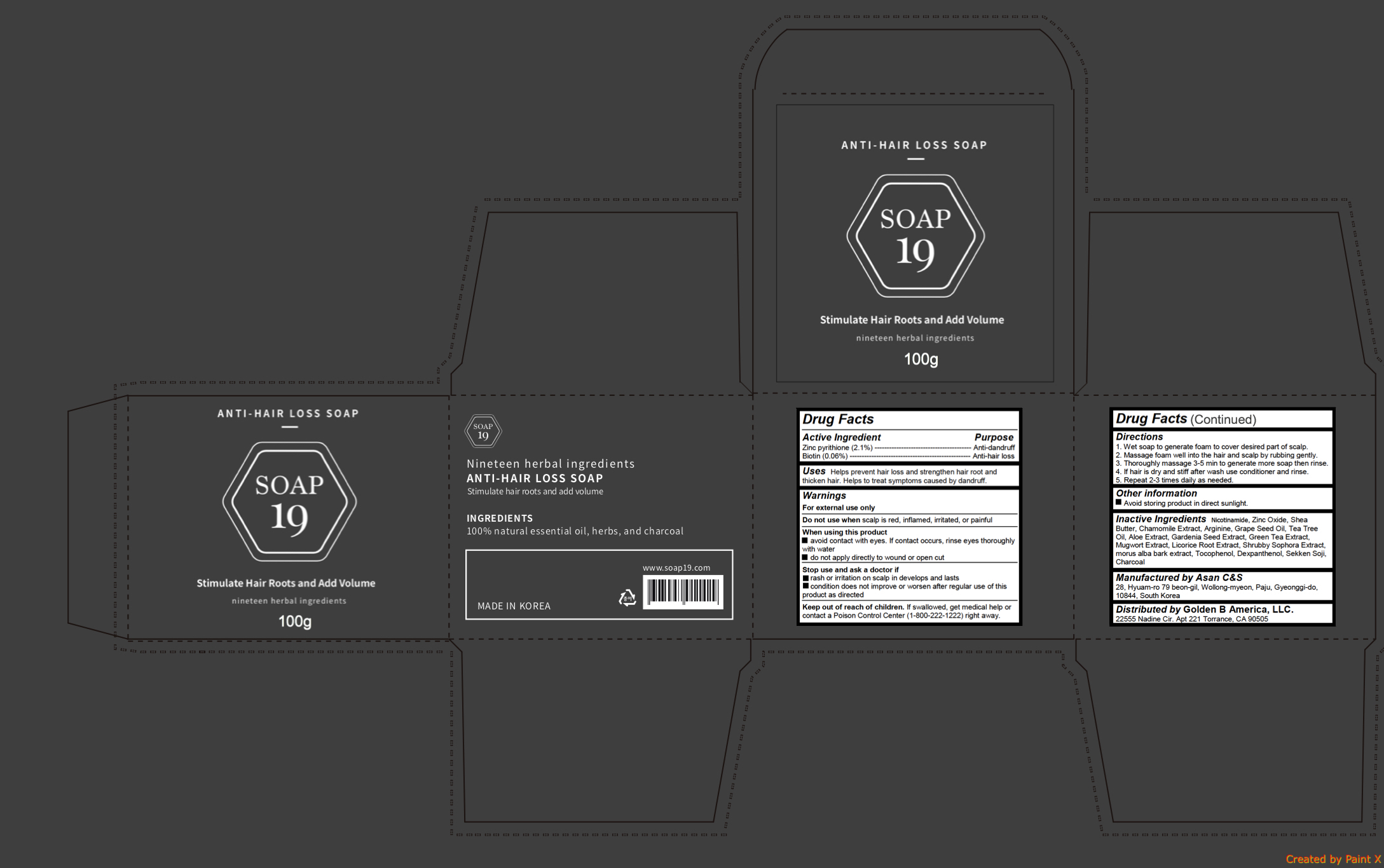
Uses
Helps prevent hair loss and strengthen hair root and thicken hair. Helps to treat symptoms caused by dandruff
Warnings
For external use only
Do not use when scalp is red, inflamed, irritated, or painful
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
- do not apply directly to wound or open cut
Stop use and ask a doctor if
- rash or irritation on scalp in develops and lasts
- condition does not improve or worsen after regular use of this product as directed
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
1. Wet soap to generate foam to cover desired part of scalp.
2. Massage foam well into the hair and scalp by rubbing gently.
3. Thoroughly massage 3-5 min to generate more soap then rinse.
4. If hair is dry and stiff after wash use conditioner and rinse.
5. Repeat 2-3 times daily as needed.
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Inactive ingredients
Nicotinamide, Zinc Oxide, Shea Butter, Chamomile Extract, Arginine, Grape Seed Oil, Tea Tree Oil, Aloe Extract, Gardenia Seed Extract, Green Tea Extract, Mugwort Extract, Licorice Root Extract, Shrubby Sophora Extract, morus alba bark extract, Tocophenol, Dexpanthenol, Sekken Soji, Charcoal