For use in the ear only.
Tilt head sideways. Place 5 to 10 drops in the ear, making sure that the tip of the applicator does not enter the ear canal. Keep drops in the ear for several minutes, either by using the included ear plugs or keeping the head tilted to the side. Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using the enclosed soft silicone bulb. Use twice daily for up to four days, or as directed by the doctor.
Warnings
Ask a doctor before use if
- you have ear drainage or discharge
- Ear pain
- Irritation or rash in the ear
- Dizziness
- An injury or perforation (hole) in the eardrum
- Recently had ear surgery
Whe using this product avoid contact with the eyes.
Stop use and ask a doctor if
- You need to use it for more than 3 days in a row.
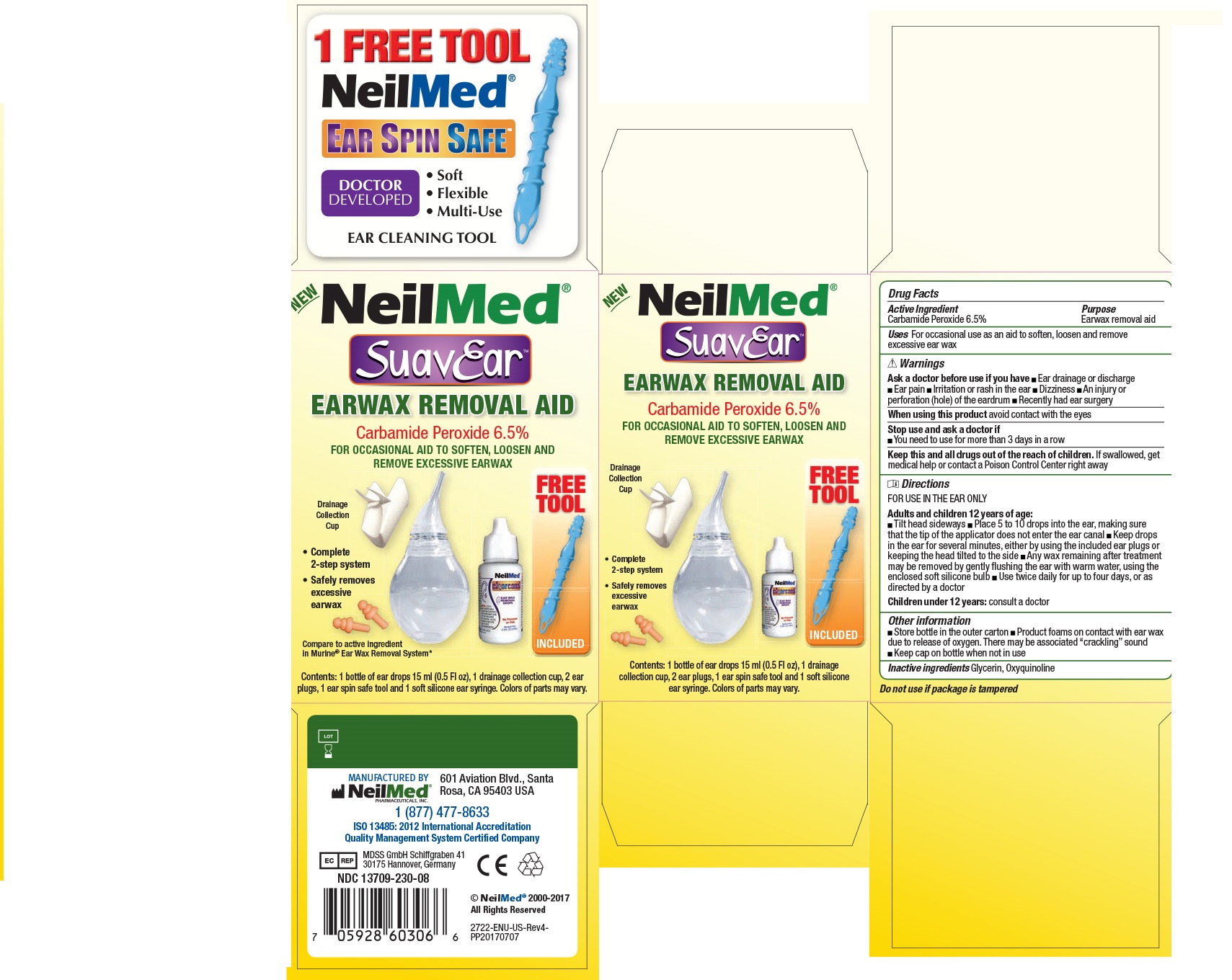 Carbamide Peroxide 6.5%
Carbamide Peroxide 6.5%