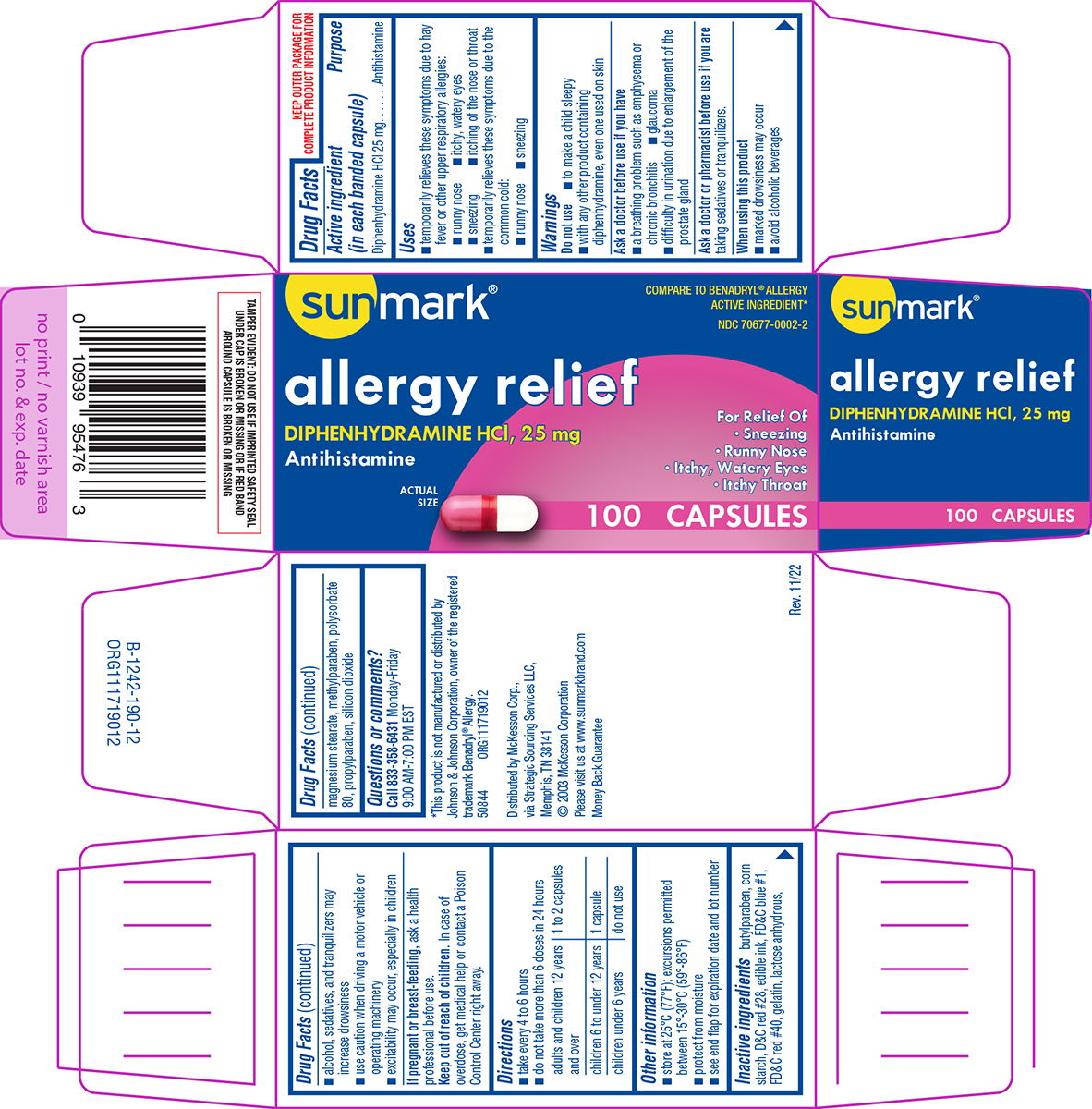
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
| adults and children 12 years and over | 1 to 2 capsules |
| children 6 to under 12 years | 1 capsule |
| children under 6 years | do not use |
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
butylparaben, corn starch, D&C red #28, edible ink, FD&C blue #1, FD&C red #40, gelatin, lactose anhydrous, magnesium stearate, methylparaben, polysorbate 80, propylparaben, silicon dioxide
Principal Display Panel
sunmark®
COMPARE TO BENADRYL® ALLERGY
ACTIVE INGREDIENT*
NDC 70677-0002-2
allergy relief
DIPHENHYDRAMINE HCl, 25 mg
Antihistamine
For Relief Of
• Sneezing
• Runny Nose
• Itchy, Watery Eyes
• Itchy Throat
ACTUAL
SIZE
100 CAPSULES
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL
UNDER CAP IS BROKEN OR MISSING OR IF RED BAND
AROUND CAPSULE IS BROKEN OR MISSING
*This product is not manufactured or distributed by
Johnson & Johnson Corporation, owner of the registered
trademark Benadryl® Allergy.
50844 ORG111719012
Distributed by McKesson Corp.,
via Strategic Sourcing Services LLC,
Memphis, TN 38141
© 2003 McKesson Corporation
Please visit us at www.sunmarkbrand.com
Money Back Guarantee
Sunmark 44-190