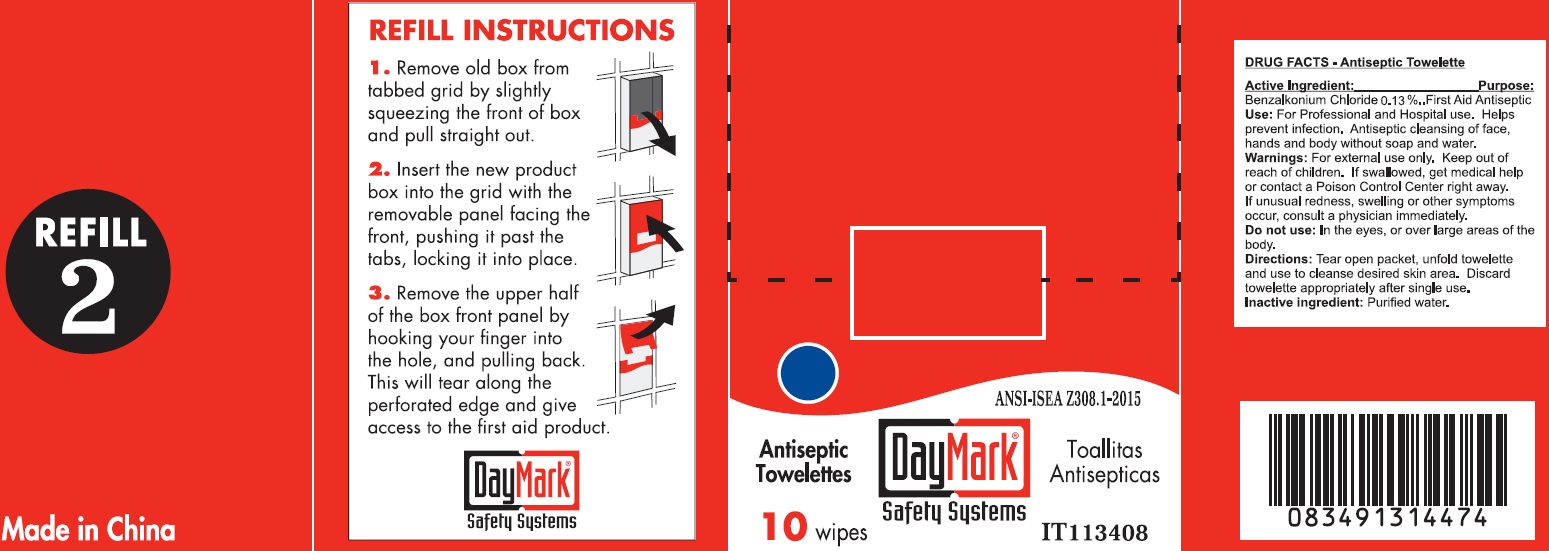
Antiseptic Towelette 10 count 49687-0011-0 DRUG FACTS
Active Ingredient:
Benzalkonium Chloride 0.13%
Purpose:
First Aid Antiseptic
Use:
For Professional and Hospital use. Helps prevent infection. Antiseptic cleansing of face, hands and body without soap and water.
Warnings:
For external use only.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. If unusual redness, swelling or other symptoms occur, consult a physician immediately.
Do not use:
In the eyes, or over large areas of the body.
Directions:
Tear open packet, unfold towelette and use to cleanse desired skin area. Discard towelette appropriately after single use.
Inactive ingredient:
Purified water.
Package Labeling:

Package Labeling:
CMC Group, Inc.