SOFTSOAP ANTIBACTERIAL WITH MOISTURIZERS CRISP CLEAN- benzalkonium chloride liquid
Colgate-Palmolive Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
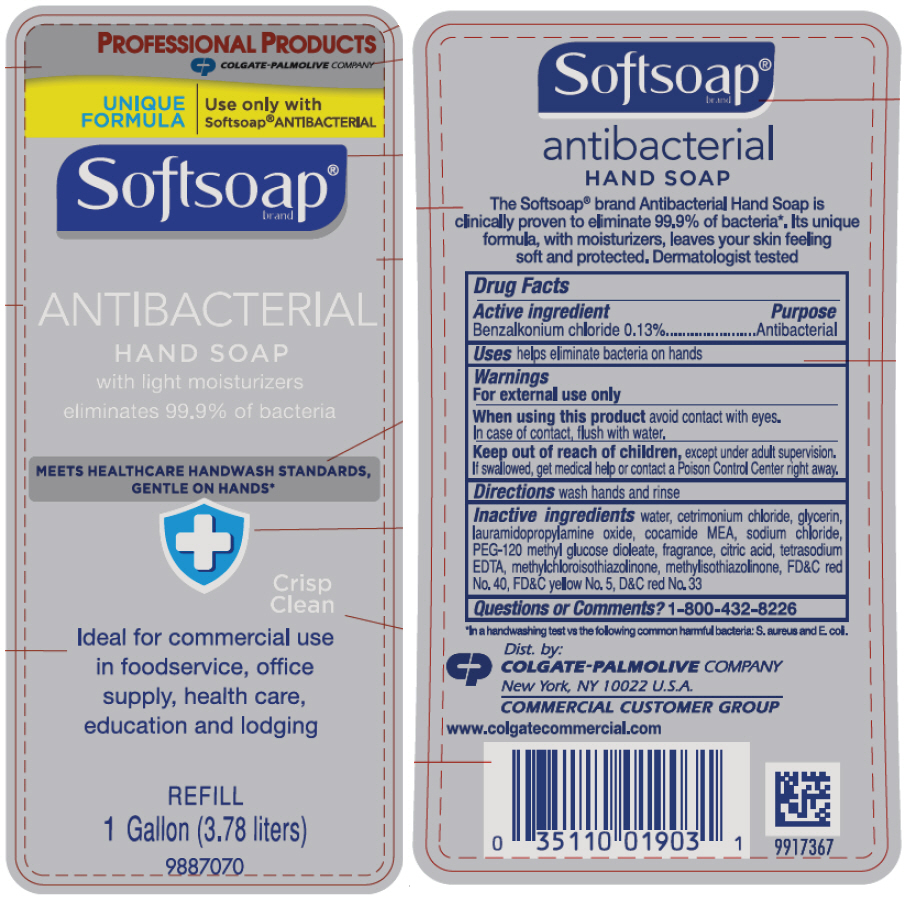
Active ingredient
Benzalkonium chloride 0.13%
Use
helps eliminate bacteria on hands
Warnings
For external use only
When using this product, avoid contact with eyes. In case of contact, flush with water.
Keep out of reach of children except under adult supervision. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
wash hands and rinse
Inactive ingredients
water, cetrimonium chloride, glycerin, lauramidopropylamine oxide, cocamide MEA, sodium chloride, PEG-120 methyl glucose dioleate, fragrance, citric acid, tetrasodium EDTA, methylchloroisothiazolinone, methylisothiazolinone, red 40, yellow 5, red 33
Questions?
1-800-255-7552
DIST. BY: COLGATE PALMOLIVE COMPANY, NEW YORK, NY 10022 USA
PRINCIPAL DISPLAY PANEL - 1.47 L Bottle Label
Softsoap®
brand
ANTIBACTERIAL
HAND SOAP WITH MOISTURIZERS
GENTLY CLEANS & PROTECTS
Crisp
Clean
USE ONLY WITH
SOFTSOAP®
ANTIBACTERIAL
UNIQUE FORMULA
REFILL
50 FL OZ
(1.56 QT) 1.47 L
9928895
PRINCIPAL DISPLAY PANEL - 3.78 liter Bottle Label
PROFESSIONAL PRODUCTS
COLGATE-PALMOLIVE COMPANY
UNIQUE
FORMULA
Use only with
Softsoap® ANTIBACTERIAL
Softsoap®
brand
ANTIBACTERIAL
HAND SOAP
with light moisturizers
eliminates 99.9% of bacteria
MEETS HEALTHCARE HANDWASH STANDARDS,
GENTLE ON HANDS*
Crisp
Clean
Ideal for commercial use
in foodservice, office
supply, health care,
education and lodging
REFILL
1 Gallon (3.78 liters)
9887070
PRINCIPAL DISPLAY PANEL - 591 ml Bottle Label
Softsoap®
brand
Antibacterial
Eliminates 99.9% of Bacteria*
HAND SOAP
REFILL
USE ONLY WITH
SOFTSOAP®
ANTIBACTERIAL
Crisp Clean
Hand Soap
20 FL OZ (591 ml)
9943737