EROS AQUA BASED- glycerin gel
MEGASOL COSMETIC GMBH
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
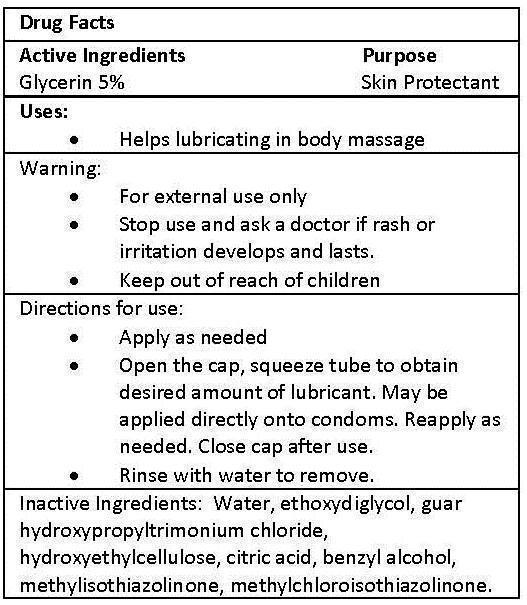
DIRECTIONS FOR USE:
- APPLY AS NEEDED
- OPEN THE CAP, SQUEEZE TUBE TO OBTAIN DESIRED AMOUNT OF LUBRICANT. MAY BE APPLIED DIRECTLY ONTO CONDOMS. REAPPLY AS NEEDED. CLOSE CAP AFTER USE.
- RINSE WITH WATER TO REMOVE.
| EROS AQUA BASED
glycerin gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - MEGASOL COSMETIC GMBH (344152855) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MEGASOL COSMETIC GMBH | 344152855 | manufacture(53389-241) | |
Revised: 10/2018
Document Id: 56436b39-d949-42e1-8bf9-cd6540dd746a
Set id: 65c9907f-f90d-4f04-aa15-deb75ab9360b
Version: 16
Effective Time: 20181011
MEGASOL COSMETIC GMBH