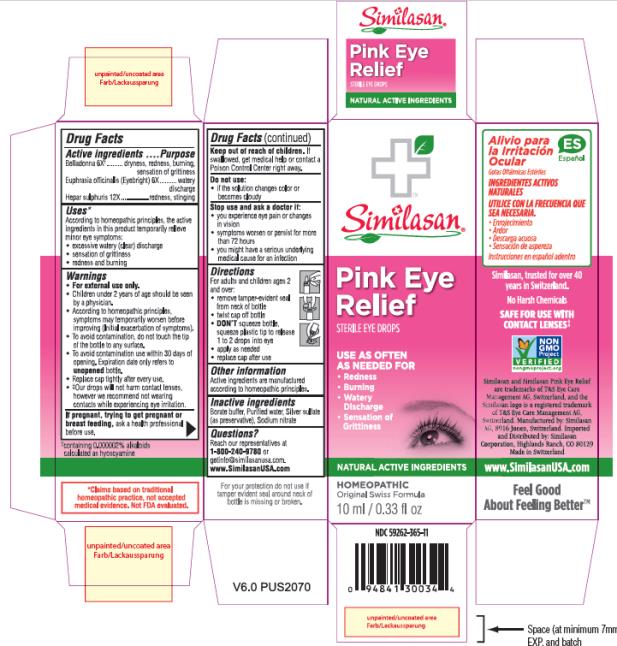
Uses*
According to homeopathic principles, the active ingredients in this product temporarily relieve minor eye symptoms:
• excessive watery (clear) discharge
• sensation of grittiness
• redness and burning
Warnings
• For external use only.
• Children under 2 years of age should be seen by a physician.
• According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms).
• To avoid contamination, do not touch the tip of the bottle to any surface.
• To avoid contamination use within 30 days of opening. Expiration date only refers to unopened bottle.
• Replace cap tightly after every use.
• ‡Our drops will not harm contact lenses, however we recommend not wearing contacts while experiencing eye irritation.
Directions
For adults and children ages 2 and over:
• remove tamper-evident seal from neck of bottle
• twist cap off bottle
• DON’T squeeze bottle, squeeze plastic tip to release 1 to 2 drops into eye
• apply as needed
• replace cap after use
Inactive ingredients
Borate buffer, Purified water, Silver sulfate (as preservative), Sodium nitrate
Questions?
Reach our representatives at 1-800-240-9780 or getinfo@similasanusa.com.
www.SimilasanUSA.com
For your protection do not use if tamper evident seal around neck of bottle is missing or broken.
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.