KINDEST KARE AIR-INFUSED FOAM ANTISEPTIC HANDRUB- alcohol liquid
SC Johnson Professional USA, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
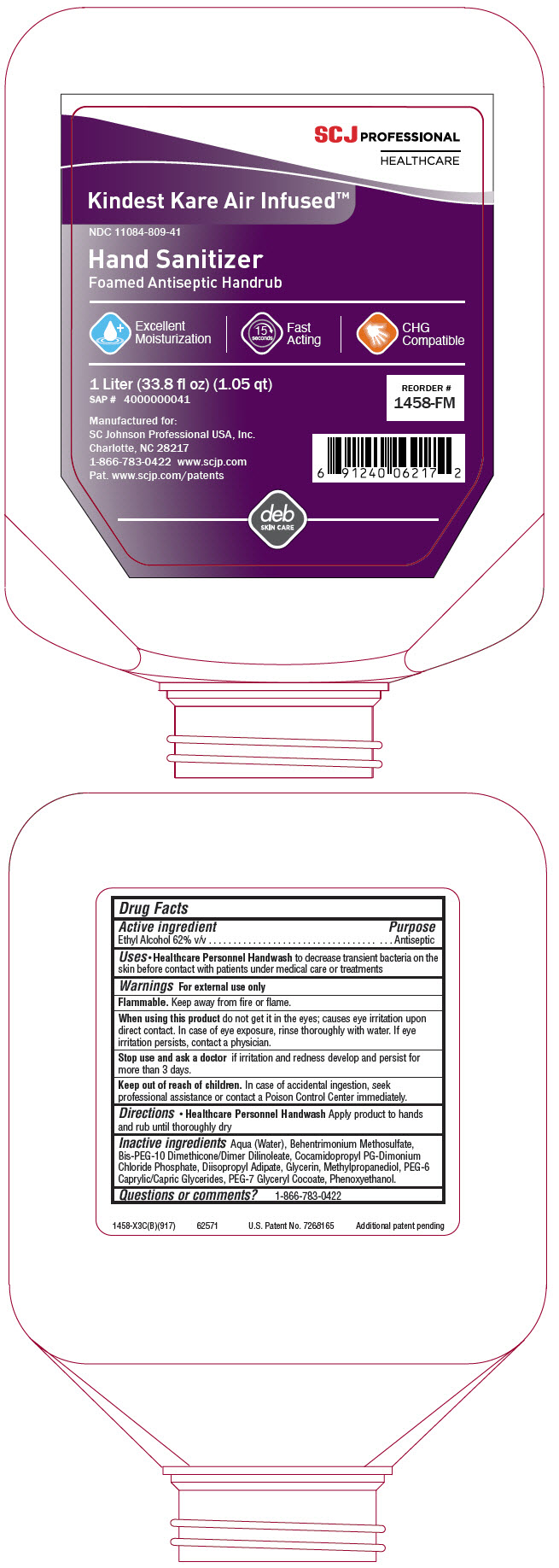
Active ingredient
Ethyl Alcohol 62% v/v
Uses
-
Healthcare Personnel Handwash to decrease transient bacteria on the skin before contact with patients under medical care or treatments
Warnings
For external use only
Flammable. Keep away from fire or flame.
When using this product do not get it in the eyes; causes eye irritation upon direct contact. In case of eye exposure, rinse thoroughly with water. If eye irritation persists, contact a physician.
Stop use and ask a doctor if irritation and redness develop and persist for more than 3 days.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
-
Healthcare Personnel Handwash Apply product to hands and rub until thoroughly dry
Inactive ingredients
Aqua (Water), Behentrimonium Methosulfate, Bis-PEG-10 Dimethicone/Dimer Dilinoleate, Cocamidopropyl PG-Dimonium Chloride Phosphate, Diisopropyl Adipate, Glycerin, Methylpropanediol, PEG-6 Caprylic/Capric Glycerides, PEG-7 Glyceryl Cocoate, Phenoxyethanol.
Questions or comments?
1-866-783-0422
PRINCIPAL DISPLAY PANEL - 1 Liter Bottle Label
SCJ PROFESSIONAL
HEALTHCARE
Kindest Kare Air Infused™
NDC 11084-809-41
Hand Sanitizer
Foamed Antiseptic Handrub
Excellent
Moisturization
15
seconds
Fast
Acting
CHG
Compatible
1 Liter (33.8 fl oz) (1.05 qt)
SAP # 4000000041
REORDER #
1458-FM
Manufactured for:
SC Johnson Professional USA, Inc.
Charlotte, NC 28217
1-866-783-0422 www.scjp.com
Pat. www.scjp.com/patents
deb
SKIN CARE
SC Johnson Professional USA, Inc.