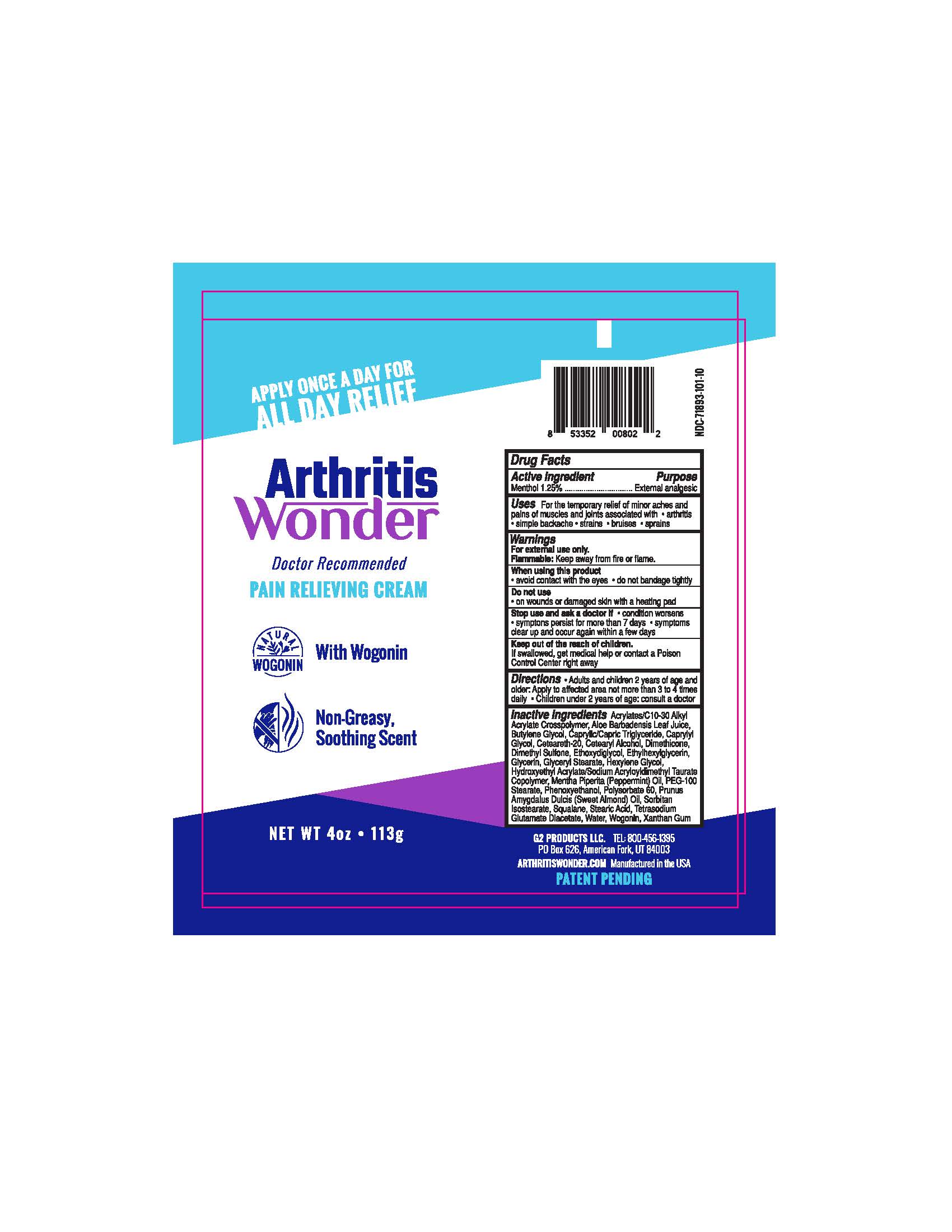
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with ● arthritis ● simple backache ● strains ● bruises ● sprains
Warnings
For external use only.
Flammable: Keep away from fire or flame.
Directions
• Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily • Children under 2 years of age: consult a doctor
Inactive ingredients:
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, Butylene Glycol, Caprylic/Capric Triglyceride, Caprylyl Glycol, Ceteareth-20, Cetearyl Alcohol, Dimethicone, Dimethyl Sulfone, Ethoxydiglycol, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Hexylene Glycol, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Mentha Piperita (Peppermint) Oil, PEG-100 Stearate, Phenoxyethanol, Polysorbate 60, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Sorbitan Isostearate, Squalane, Stearic Acid, Tetrasodium Glutamate Diacetate, Water, Wogonin, Xanthan Gum