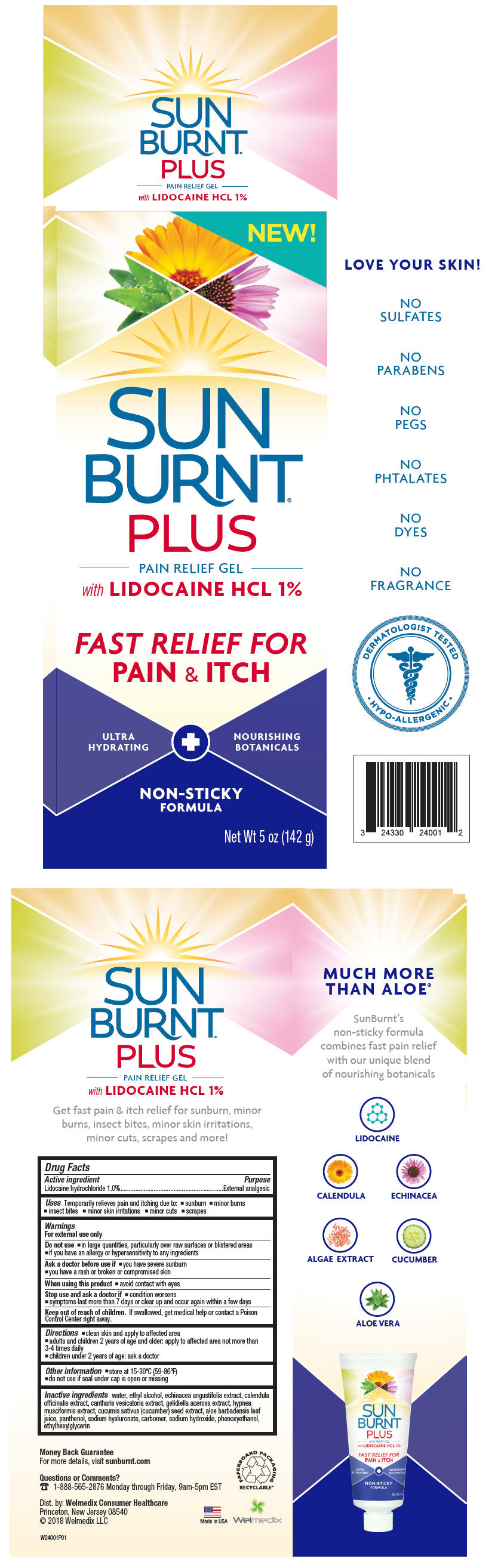
SUNBURNT PLUS PAIN RELIEF- lidocaine hydrochloride gel
Welmedix LLC dba Welmedix Consumer Healthcare
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Lidocaine hydrochloride 1.0%
Purpose
External analgesic
Uses
Temporarily relieves pain and itching due to:
- sunburn
- minor burns
- insect bites
- minor skin irritations
- minor cuts
- scrapes
Warnings
For external use only
Do not use
- in large quantities, particularly over raw surfaces or blistered areas
- if you have an allergy or hypersensitivity to any ingredients
Ask a doctor before use if
- you have severe sunburn
- you have a rash or broken or compromised skin
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean skin and apply to affected area
- adults and children 2 years of age and older: apply to affected area not more than 3-4 times daily
- children under 2 years of age: ask a doctor
Other information
- store at 15-30ºC (59-86ºF)
- do not use if seal under cap is open or missing
Inactive ingredients
water, ethyl alcohol, echinacea angustifolia extract, calendula officinalis extract, cantharis vesicatoria extract, gelidiella acerosa extract, hypnea musciformis extract, cucumis sativus (cucumber) seed extract, aloe barbadensis leaf juice, panthenol, sodium hyaluronate, carbomer, sodium hydroxide, phenoxyethanol, ethylhexylglycerin
Questions or Comments?
1-888-565-2876 Monday through Friday, 9am-5pm EST
Dist. by: Welmedix Consumer Healthcare
Princeton, New Jersey 08540
PRINCIPAL DISPLAY PANEL - 142 g Tube Carton
NEW!
SUN
BURNT®
PLUS
PAIN RELIEF GEL
with LIDOCAINE HCL 1%
FAST RELIEF FOR
PAIN & ITCH
ULTRA
HYDRATING
NOURISHING
BOTANICALS
NON-STICKY
FORMULA
Net Wt 5 oz (142 g)