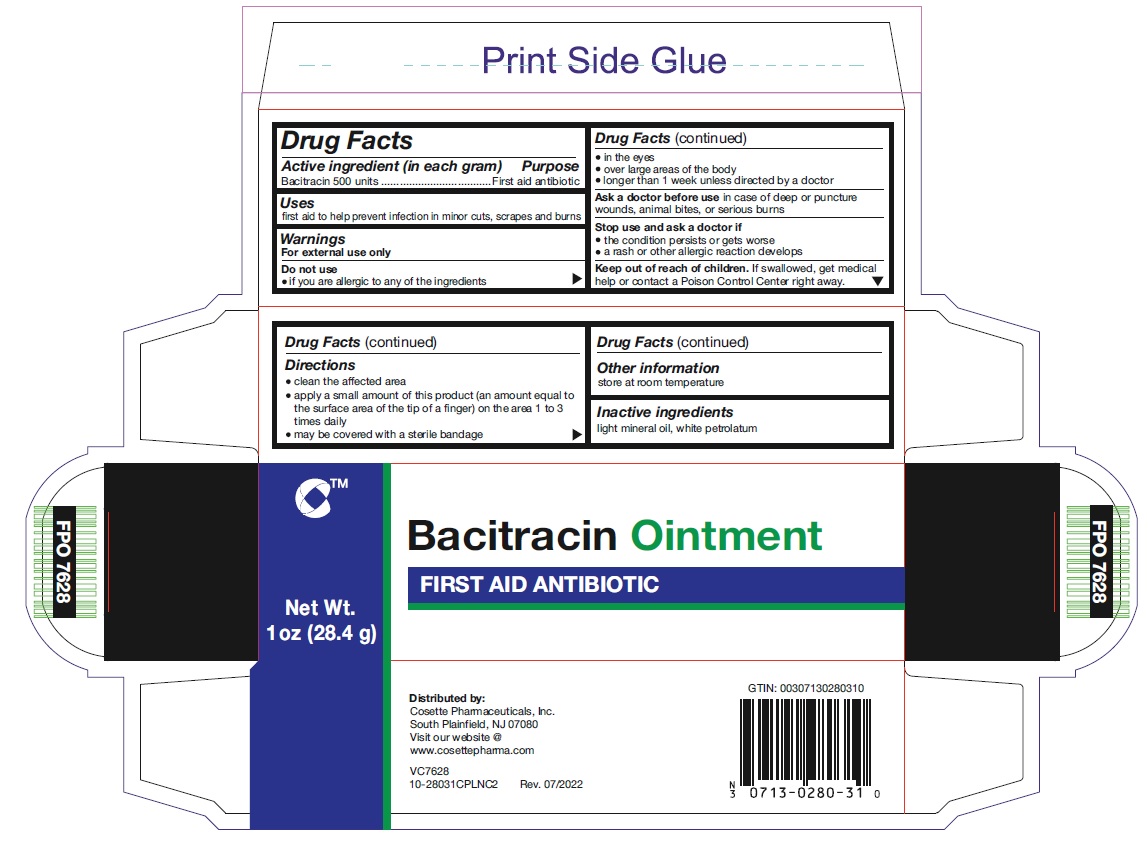
WARNINGS
For external use only
Do not use
• if you are allergic to any of the ingredients
• in the eyes
• over large areas of the body
• longer than 1 week unless directed by a doctor
Ask a doctor before usein case of deep or puncture wounds, animal bites, or serious burns
Stop use and ask a doctor if
• the condition persists or gets worse
• a rash or other allergic reaction develops
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.