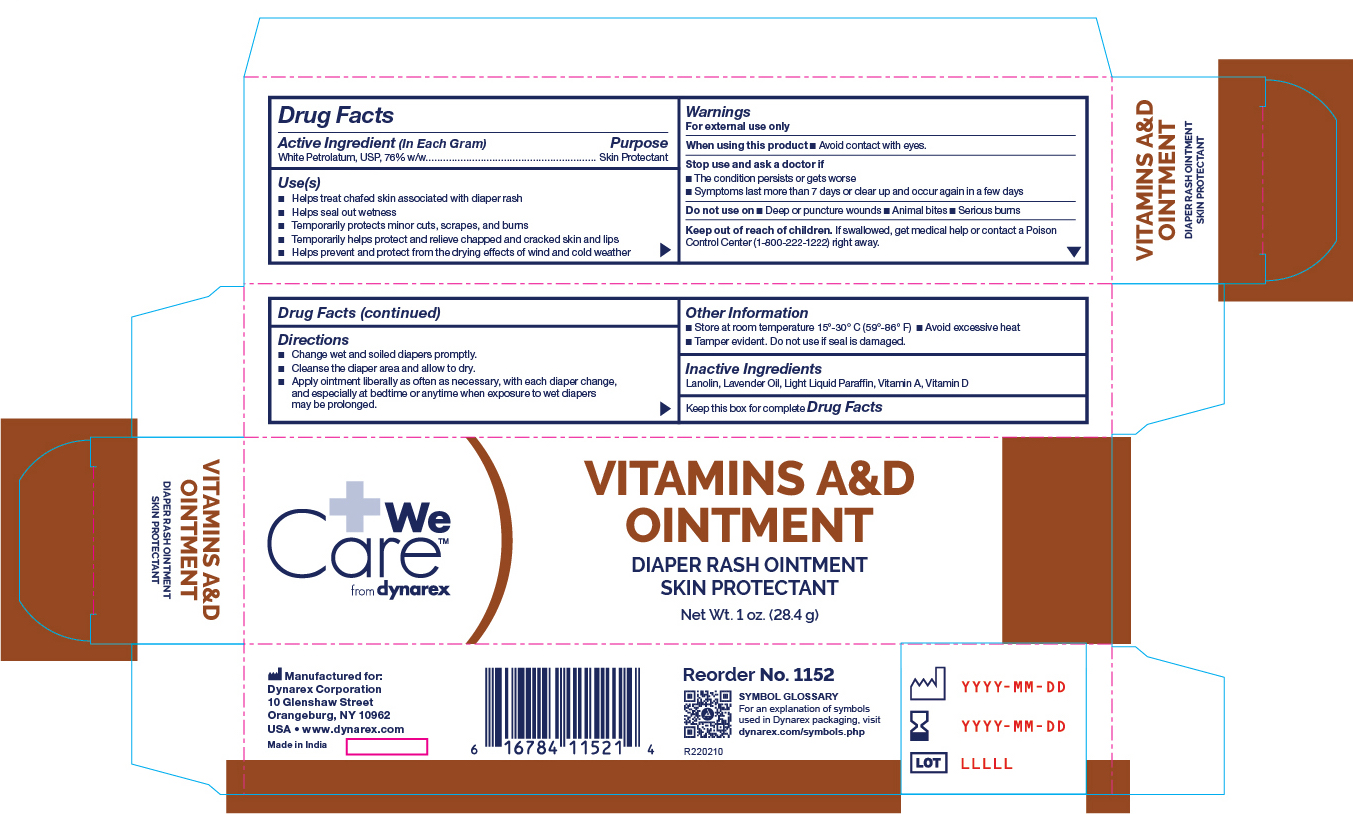
Uses: Vitamin_A_D
- Helps treat and prevent diaper rash
- Helps seal out wetness
- Temporarily protects minor * cuts * scrapes * burns
- Temporarily helps protect and help relieve chapped and cracked skin and lips
- Helps prevent and protect from the drying effects of wind and cold weather
- Helps prevent and protect chafed skin or minor skin irritations associated with diaper rash
- With each diaper change, especially at bedtime when exposure to wet diapers may be prolonged
Stop use and ask a doctor if:
- If condition worsens
- symptoms last more than 7 days or clear up and occur again in a few days
Keep out of reach of children
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions for diaper rash:
- Change wet and soiled diapers promptly
- Cleanse the diaper area and allow to dry
- Apply as needed
Other information:
- Store at room temperature 15 deg C to 30 deg C 59 deg F to 86 deg F
- Avoid excessive heat
Inactive Ingredients
Inactive Ingredients: Lanolin, Lavender Oil, Light Liquid Paraffin, Vitamin A, Vitamin D