Active ingredient
Povidone-iodine USP 10%
Uses
- • antiseptic skin preparation
- • helps reduce bacteria that can potentially cause skin infections
Warnings
For external use only
Do not use
- • if allergic to iodine
- • in the eyes
- • on children less than 3 years old
Ask a doctor before use if injuries are
- • deep or puncture wounds
- • serious burns
Stop use and ask a doctor if
- • redness, irritation, swelling or pain persists or increases
- • infection occurs
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
Avoid excessive heat. Store at room temperature.
Directions
NASAL APPLICATION:
|

|
- Use a tissue to clean the inside of both nostrils, including the inside tip of nostril. Discard.
|
|
|
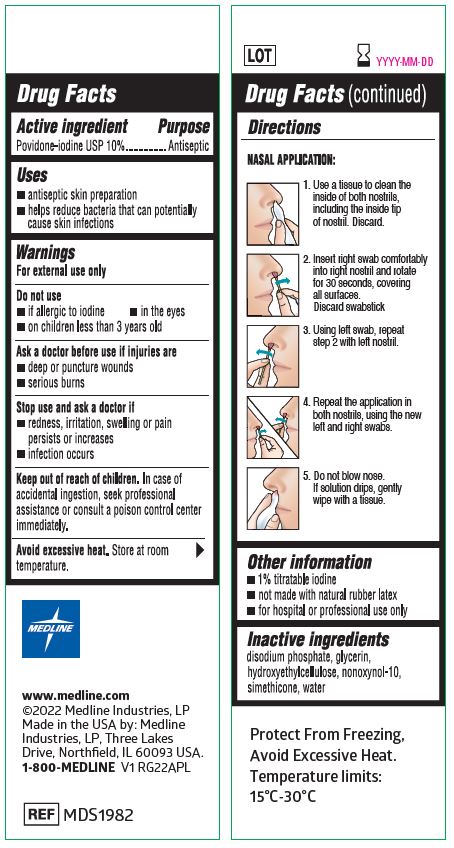
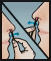
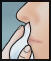
- Insert swab comfortably into one nostril and rotate for 30 seconds, covering all surfaces. Discard swabstick.
|
|
|
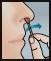
- Using a 2nd swab, repeat step 2 with the other nostril.
|
|
|
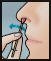
- Repeat the application in both nostrils, using the 3rd and 4th swabs.
|
|
|
- Do not blow nose. If solution drips, gently wipe with a tissue.
|
Other information
- • 1% titratable iodine
- • not made with natural rubber latex
- • for hospital or professional use only
Inactive ingredients
disodium phosphate, glycerin, hydroxyethylcellulose, nonoxynol-10, simethicone, water
Manufacturing Information
Manufactured by:
Medline Industries, LP
Three Lakes Drive, Northfield IL 60093 USA
Made in USA
1-800-MEDLINE (633-5463)
REF: MDS1982
V1 RG22APL
Package Label