CHILDRENS SILFEDRINE- pseudoephedrine hydrochloride liquid
Lannett Company, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
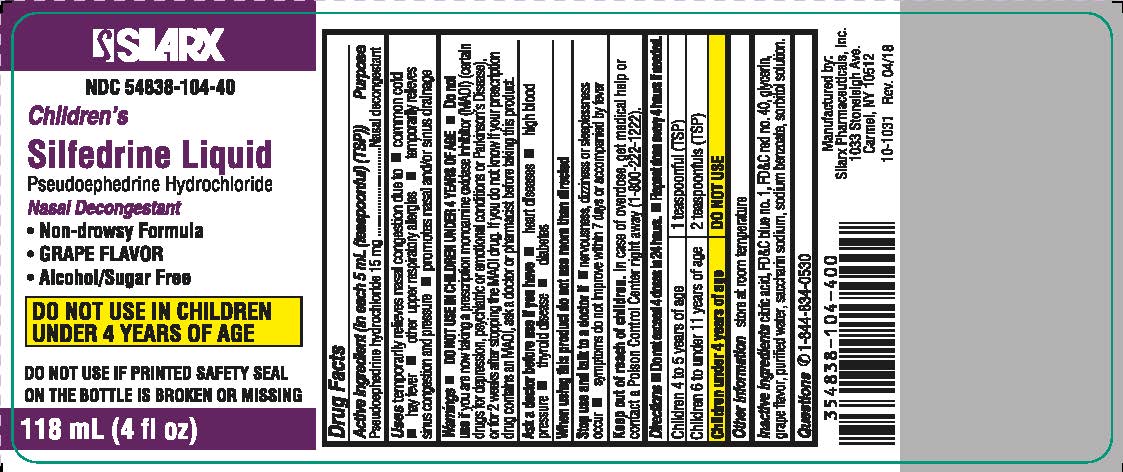
Children's Silfedrine Liquid
Uses temporarily relieves nasal congestion due to
- common cold
- hay fever
- other upper respiratory allergies
- temporarily relieves sinus congestion and pressure
- promotes nasal and/or sinus drainage
Warnings
DO NOT USE IN CHILDREN UNDER 4 YEARS OF AGE
- Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Directions
- Do not exceed 4 doses in 24 hours.
- Repeat dose every 4 hours if needed.
| Children 6 to 11 years of age | 2 teaspoonfuls (TSP) |
| Children 4 to 5 years of age | 1 teaspoonful(TSP) |
| Children under 4 years of age | DO NOT USE |
Other information
store at room temperature
Inactive ingredients
citric acid, FD&C blue no. 1, FD&C red no. 40, glycerin, grape flavor, purified water, saccharin sodium, sodium benzoate, sodium citrate, sorbitol solution.
| CHILDRENS SILFEDRINE
pseudoephedrine hydrochloride liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Lannett Company, Inc. (002277481) |
Revised: 4/2018
Document Id: b258710c-7357-42e0-8cdf-35e3e719d2f3
Set id: 62722653-d91a-4822-b9fa-1d95269d1ec6
Version: 16
Effective Time: 20180420
Lannett Company, Inc.