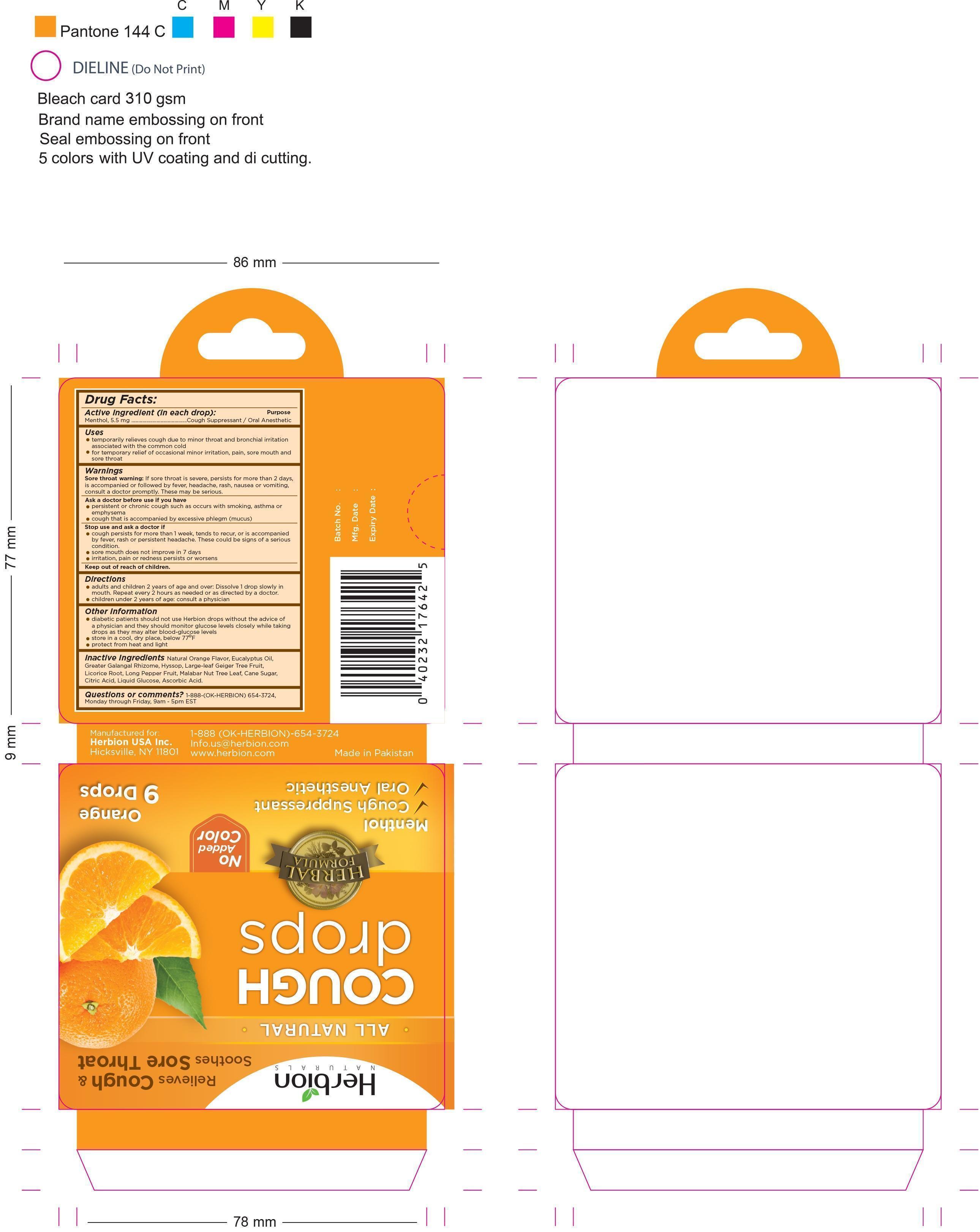
Uses:
- temporarily relieves cough due to minor throat and bronchial irritation associated with the common cold
- for temporary relief of occasional minor irritation, pain, sore mouth and sore throat
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.These may be serious
Ask a doctor before use if you have:
- persistent or chronic cough such as occurs with smoking, asthma or emphysema
- cough that is accompanied by excessive phlegm (mucus)
Stop use and ask a doctor if:
- cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition
- sore mouth does not improve in 7 days
- irritation, pain or redness persists or worsens
Directions:
- adults and children 2 years of age and over Dissolve 1 drop slowly in mouth. Repeat every 2 hours as needed or as directed by a doctor
- children under 2 years of age: consult a physician
Other Information:
- diabetic patients should not use Herbion drops without the advice of a physician and they should monitor glucose levels closely while taking drops as they may alter blood-glucose levels
- store in a cool, dry place, below 77°F
- protect from heat and light