MED-NAP STERILE ALCOHOL PREP PADS- isopropyl alcohol swab
Med-Nap LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
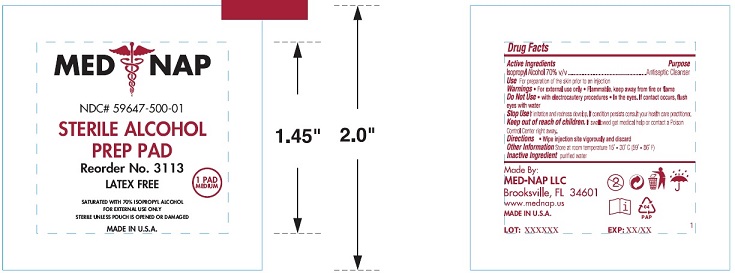
Isopropyl Alcohol 70%
Purpose
Antiseptic Cleanser
Use For preparation of the skin prior to an injection
Warnings● For external use only ● Flammable, keep away from fire or flame
Do Not Use ● with electrocautery procedures ● In the eyes. If contact occurs, flush
eyes with water
Slop Use If irritation or redness develop. If condition persists consult your health care practitioner.
Keep out of reach of children. If swallowed get medical help or consult a Poison
Control Center right away.
Directions ● Wipe injection site vigorously and discard
Other Information Store at room temperature 15ο - 30ο C (59ο - 86ο F)
Inactive Ingredient purified water
Product Label
MED NAP
NDC# 59647-500-01
STERILE ALCOHOL
PREP PAD
Reorder No. 3113
LATEX FREE
1 PAD
MEDIUM
SATURATED WITH 70% ISOPROPYL ALCOHOL
FOR EXTERNAL USE ONLY
STERILE UNLESS POUCH IS OPENED OR DAMAGED
MADE IN U.S.A.
Made By:
MED-NAP LLC
Brooksville, FL 34601
www.mednap.us
MADE IN U.S.A.
LOT: XXXXXX EXP: XX/XX 1

res

