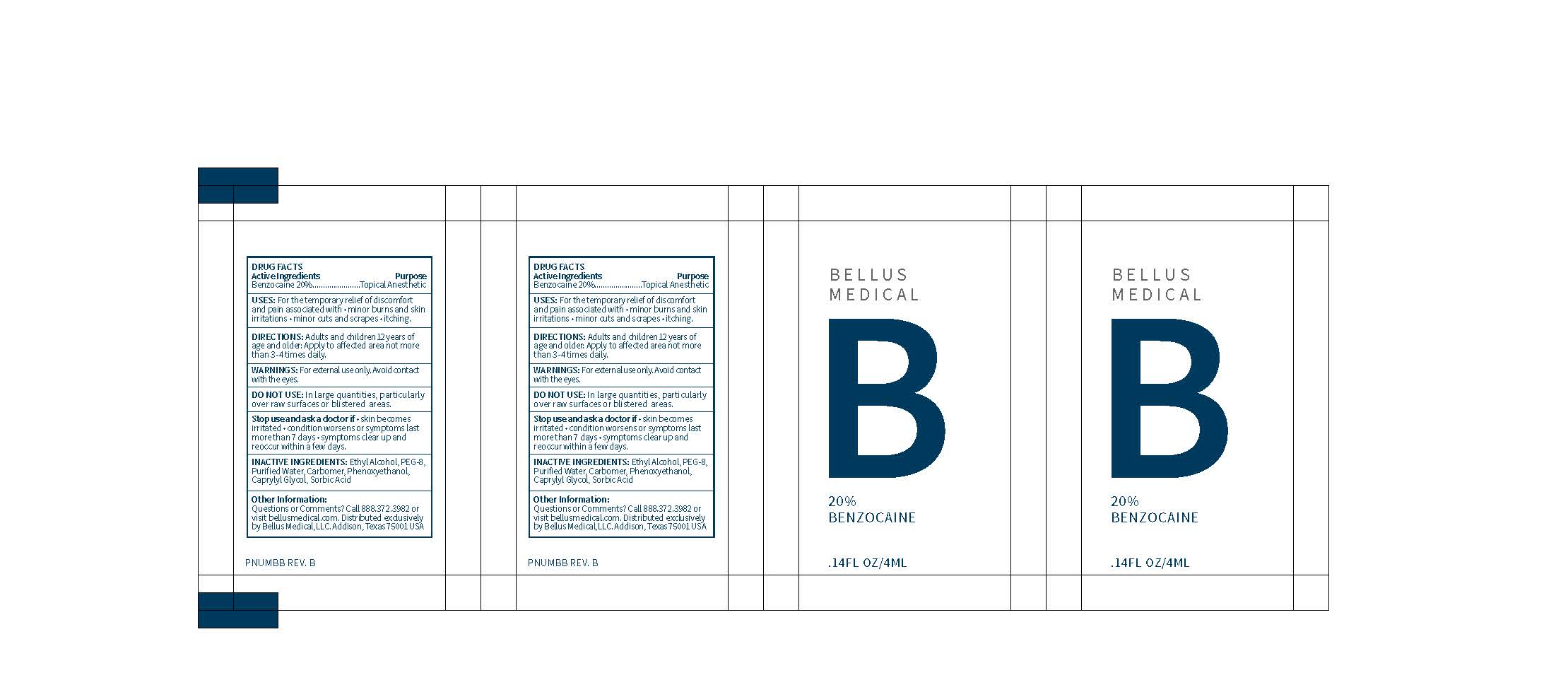
USES: For the temporary relief of discomfort and pain associated with
- minor burns and skin irritations
- minor cuts and scrapes
- itching
DIRECTIONS: Adults and children 12 years of age and older: Apply to affected area not more than 3-4 times daily.
For external use only. Avoid contact with the eyes.
Stop use and ask a doctor if:
- Skin becomes irritated
- Condition worsens or symptoms last more than 7 days
- Symptoms clear up and reoccur within a few days
DO NOT USE: in large quantities, particularly over raw surfaces or blistered areas.