MOTION SICKNESS LESS DROWSY FORMULA- meclizine hcl tablet
Strategic Sourcing Services LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
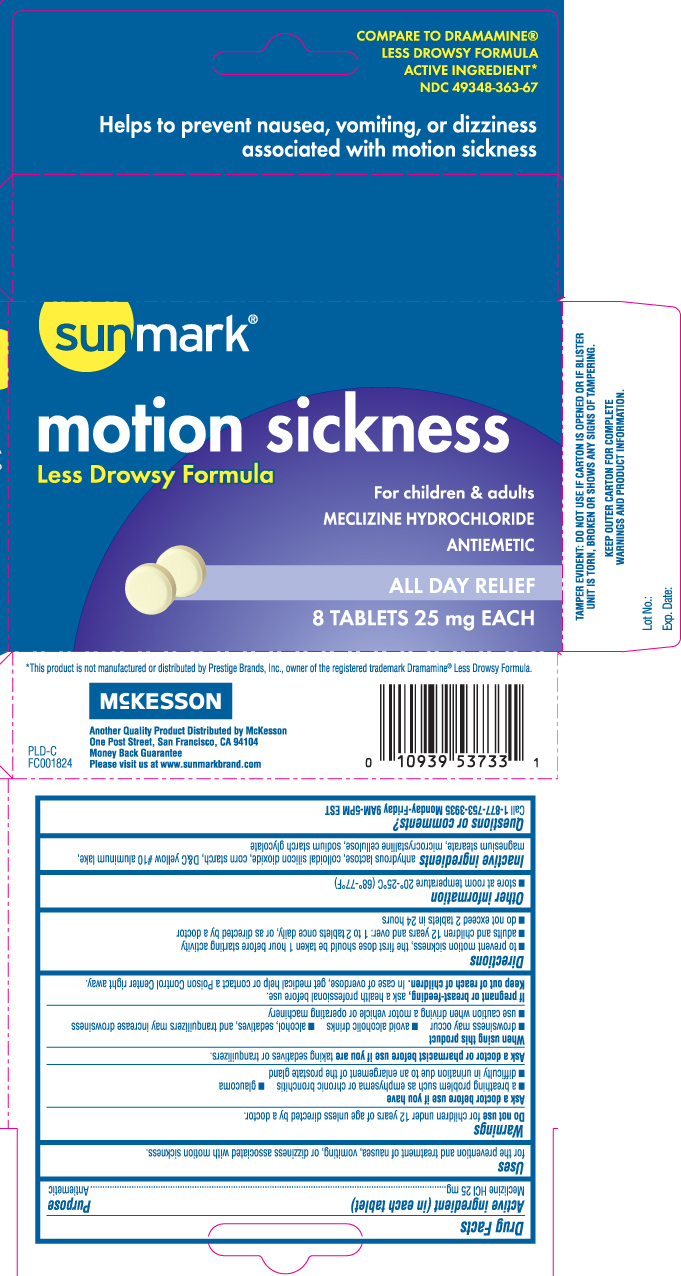
Drug Facts
Uses
for the prevention and treatment of nausea, vomiting, or dizziness associated with motion sickness.
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to an enlargement of the prostate gland
Directions
- to prevent motion sickness, the first dose should be taken 1 hour before starting activity
- adults and children 12 years and over: 1 to 2 tablets once daily, or as directed by a doctor
- do not exceed 2 tablets in 24 hours
Inactive ingredients
anhydrous lactose, colloidal silicon dioxide, corn starch, D&C yellow #10 aluminium lake, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
Principal Display Panel
COMPARE TO DRAMAMINE® LESS DROWSY FORMULA ACTIVE INGREDIENT*
Helps to prevent nausea, vomiting, or dizziness associated with motion sickness
Motion Sickness
Less Drowsy Formula
For children & adults
MECLIZINE HYDROCHLORIDE
ANTIEMETIC
ALL DAY RELIEF
TABLETS 25 mg EACH
*This product is not manufactured or distributed by Prestige Brands, Inc., owner of the registered trademark Dramamine® Less Drowsy Formula.
Another Quality Product Distributed by McKesson
One Post Street, San Francisco, CA 94104
Please visit us at www.sunmarkbrand.com
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| MOTION SICKNESS
LESS DROWSY FORMULA
meclizine hcl tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Strategic Sourcing Services LLC (116956644) |