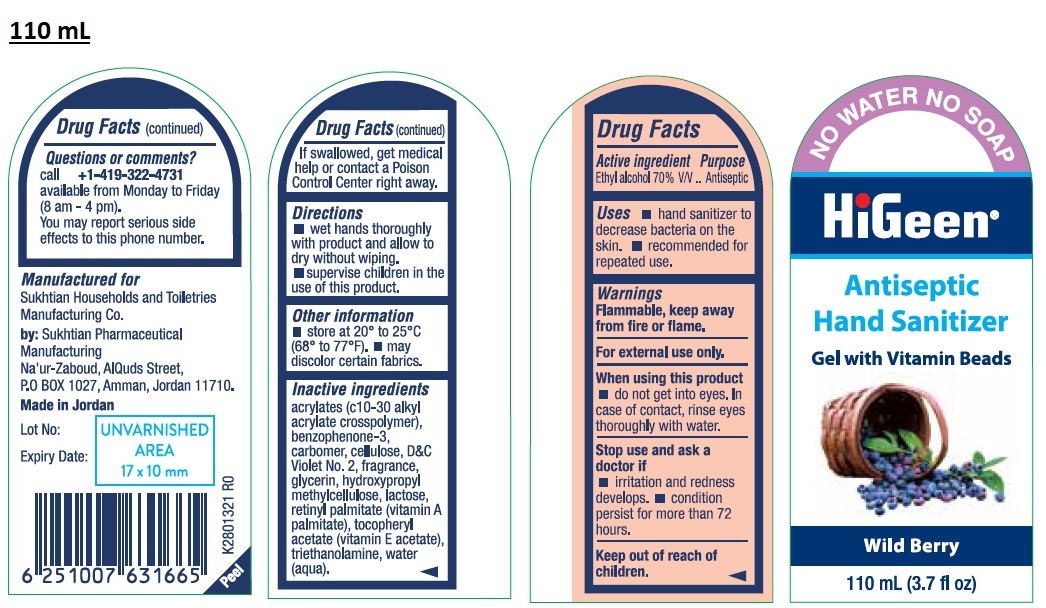
HIGEEN ANTISEPTIC HAND SANITIZER GEL WITH VITAMIN BEADS WILD BERRY- ethyl alcohol gel
SUKHTIAN-HTM ( HOUSEHOLDS & TOILETRIES MFG.CO)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Ethyl alcohol 70% V/V
Uses
• hand sanitizer to decrease bacteria on the skin. • recommended for repeated use.
Warnings
Flammable, keep away from fire or flame.
For external use only.
When using this product
• do not get into eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctor if
• irritation and redness develops. • condition persist for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• wet hands thoroughly with product and allow to dry without wiping.
• supervise children in the use of this product.
Other information
• store at 20° to 25°C (68° to 77°F). • may discolor certain fabrics.
Inactive ingredients
acrylates (c10-30 alkyl acrylate crosspolymer), benzophenone-3, carbomer, cellulose, D&C Violet No. 2, fragrance, glycerin, hydroxypropyl methylcellulose, lactose, retinyl palmitate (vitamin A palmitate), tocopheryl acetate (vitamin E acetate), triethanolamine, water (aqua).
Questions or comments?
call +1-419-322-4731
available from Monday to Friday (8 am – 4 pm).
You may report serious side effects to this phone number.
NO WATER NO SOAP
Manufactured for
Sukhtian Households and Toiletries Manufacturing Co.
by: Sukhtian Pharmaceutical Manufacturing
Na'ur-Zaboud, AlQuds Street,
P.O BOX 1027, Amman, Jordan 11710.
Made in Jordan
Packaging