Uses
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
Directions
- use only with enclosed dosing cup
| adults and children 6 years and over | 1 teaspoonful (5 mL) or 2 teaspoonfuls (10 mL) once daily depending upon severity of symptoms; do not take more than 2 teaspoonfuls (10 mL) in 24 hours. |
| adults 65 years and older | 1 teaspoonful (5 mL) once daily; do not take more than 1 teaspoonful (5 mL) in 24 hours. |
| children 2 to under 6 years of age | 1/2 teaspoonful (2.5 mL) once daily. If needed, dose can be increased to a maximum of 1 teaspoonful (5 mL) once daily or 1/2 teaspoonful (2.5 mL) every 12 hours. Do not give more than 1 teaspoonful (5 mL) in 24 hours. |
| children under 2 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Inactive ingredients
artificial grape flavor, glacial acetic acid, glycerin, methylparaben, natural and artificial banana flavor, propylene glycol, propylparaben, purified water, sodium acetate (anhydrous), sucrose
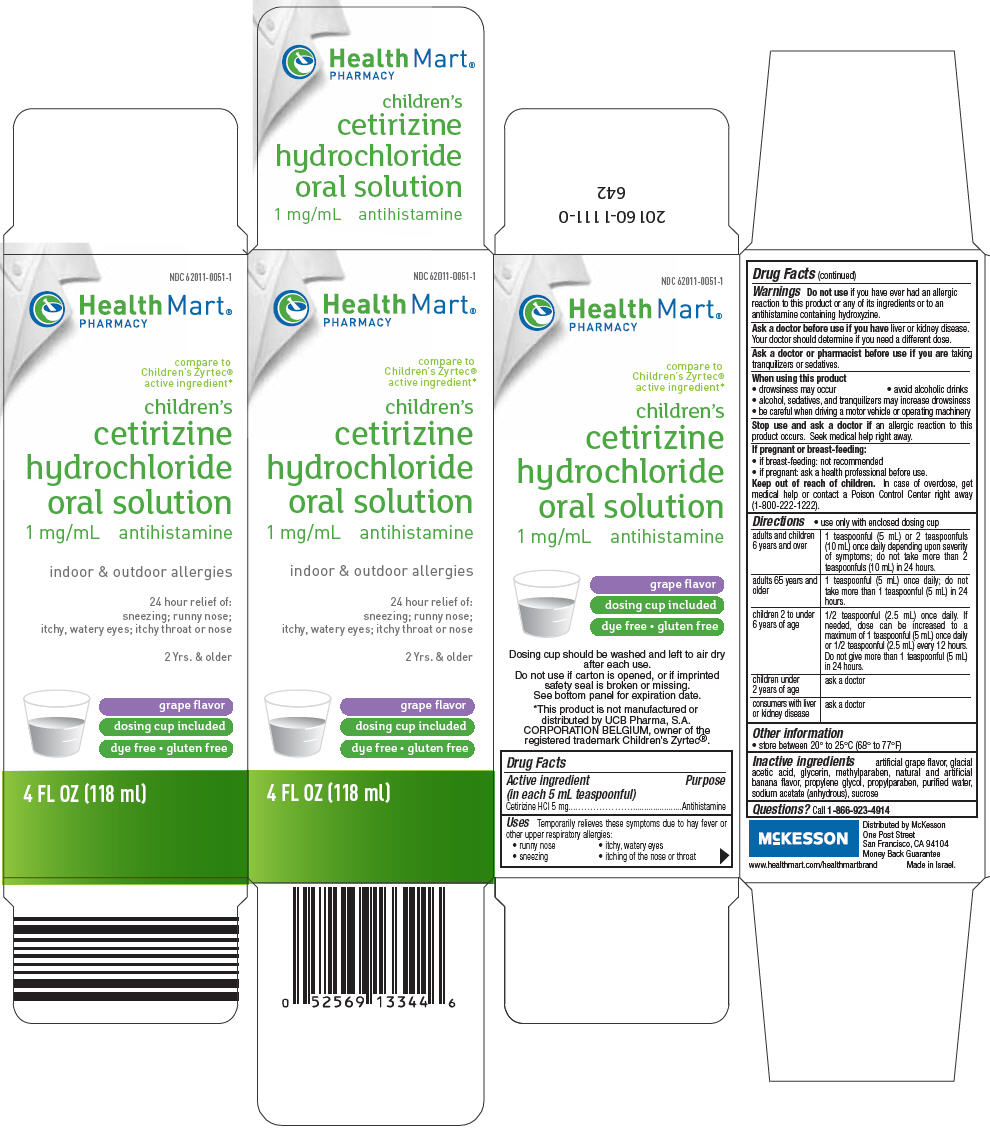
PRINCIPAL DISPLAY PANEL - 118 ml Bottle Carton
NDC 62011-0051-1
Health Mart®
PHARMACY
compare to
Children's Zyrtec®
active ingredient*
children's
cetirizine
hydrochloride
oral solution
1 mg/mL antihistamine
indoor & outdoor allergies
24 hour relief of:
sneezing; runny nose;
itchy, watery eyes; itchy throat or nose
2 Yrs. & older
grape flavor
dosing cup included
dye free • gluten free
4 FL OZ (118 ml)