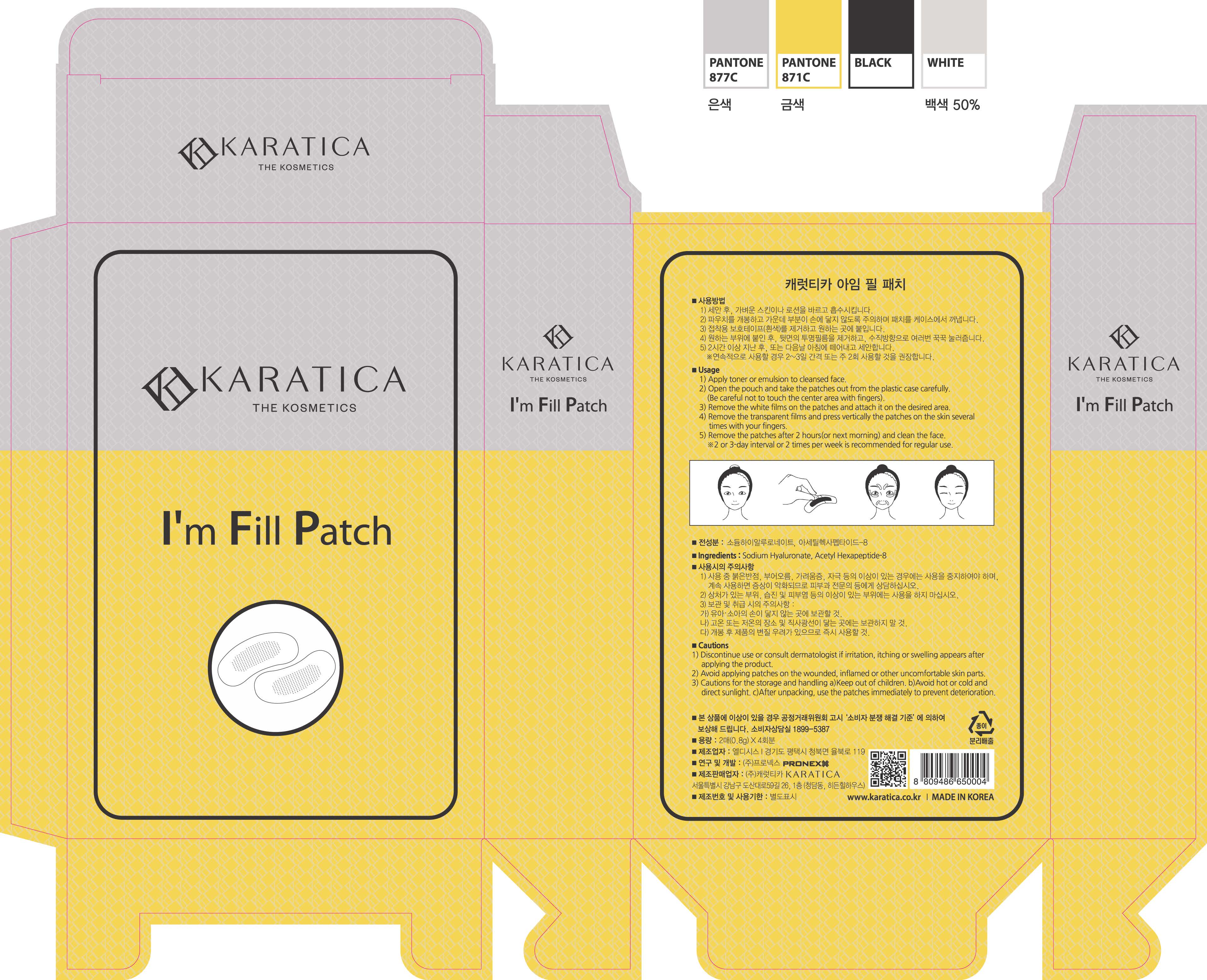
1) Apply toner or emulsion to cleansed face.
2) Open the pouch and take the patches out from the plastic case carefully.
(Be careful not to touch the center area with fingers.)
3) Remove the white films on the patches and attach it on the desired area.
4) Remove the transparent films and press vertically the patches on the skin several times with your fingers.
5) Remove the patches after 2 hours (or next morning) and clean the face.
*2 or 3 day interval or 2 times per week is recommended for regular use.
1) Discontinue use or consult dermatologist if irritation, itching or swelling appears after applying the product.
2) Avoid applying patches on the wounded, inflamed or other uncomfortable skin part.
3) Cautions for the storage and handling a) Keep out of children. B) Avoid hot or cold and direct sunlight. c) After unpacking, use the patches immediately to prevent deterioration.