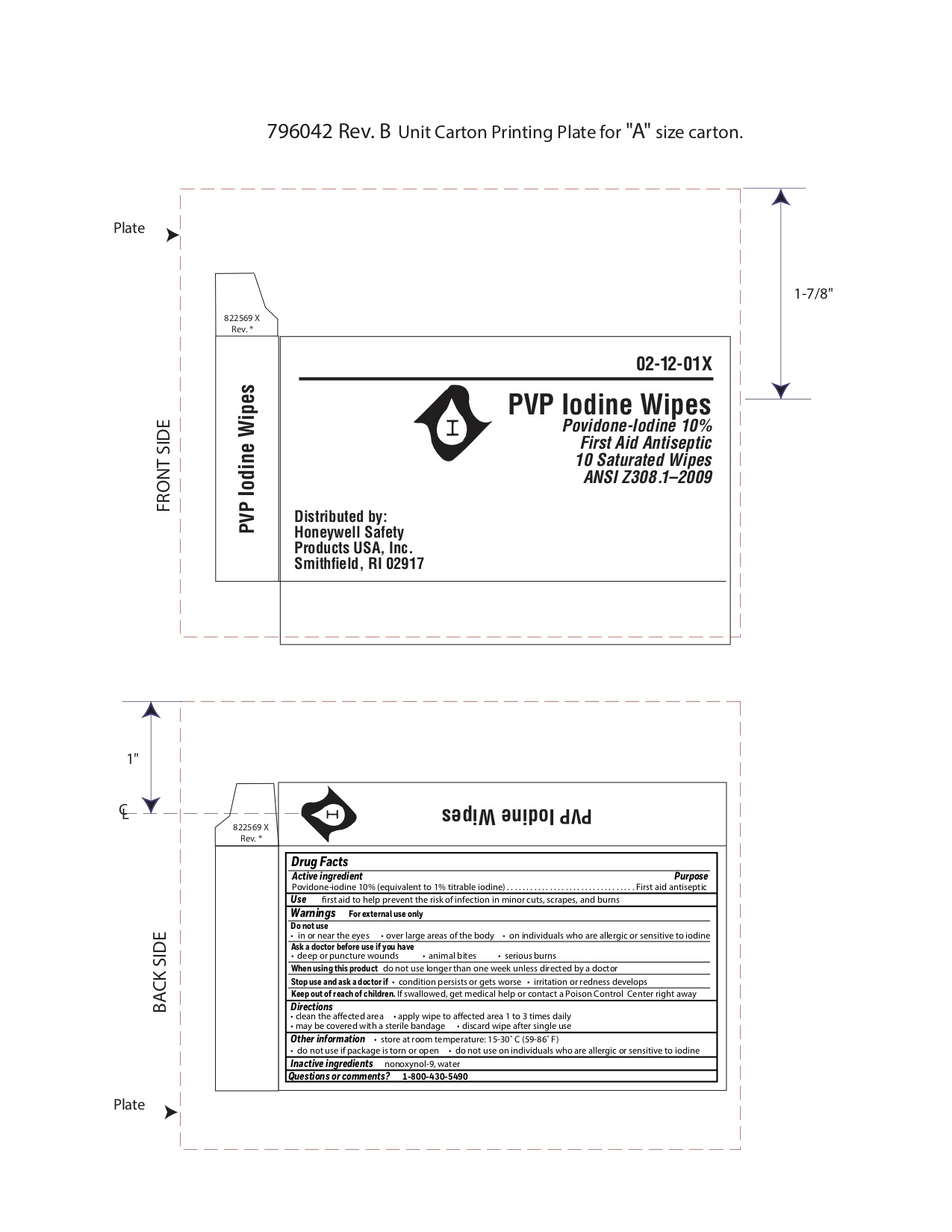
Active ingredient
Povidone-iodine 10%
(equivalent to 1% titratable iodine)
Purpose
First aid antiseptic
Uses
- first aid antiseptic to help prevent infection in minor cuts, scrapes and burns
Warnings
For external use only.
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens or persists for more than 72 hours
- irritation and redness develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the affected area
- apply1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
- discard wipe after single use
Other information
- do not use on individuals who are allergic or sensitive to iodine
- store at controlled temperature 59-86ºF (15-30ºC)
- do not use if pouch is open or torn
Inactive ingredients
nonoxynol 9, water
Principal Display Panel
PVPWipe.jpg
North PVP Iodine Wipe

Honeywell PVP Wipes