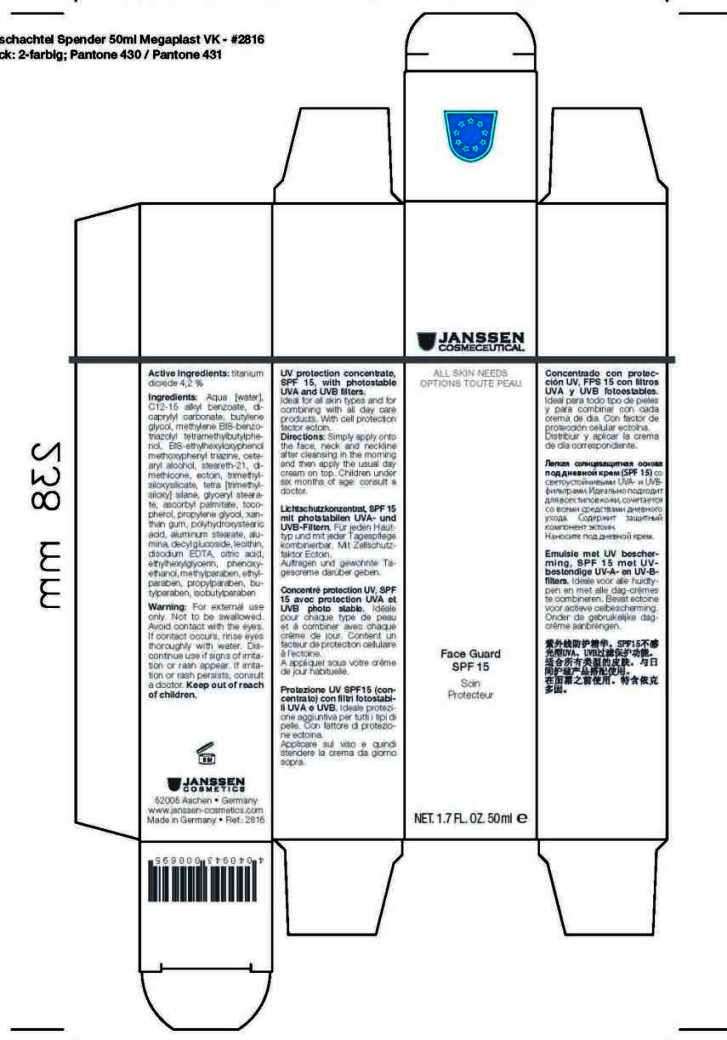
Inactive Ingredients
Aqua [water], C12-15 alkyl benzoate, dicaprylyl carbonate, butylenes glycol, methylene BIS-benzotriazolyl tetramethylbutylphenol, BIS-ethylhexyloxyphenol methoxyphenyl triazine, cetearyl alcohol, steareth-21, dimethicone, ectoin, trimethylsiloxysilicate, tetra [trimethylsiloxy] silane, glyceryl stearate, ascorbyl palmitate, tocopherol, propylene glycol, xanthan gum, polyhydroxystearic acid, aluminum stearate, alumina, decyl glucoside, lecithin, disodium EDTA, citric acid, ethylhexylglycerin, phenoxyethanol, methylparaben, ethylparaben, propylparaben, butylparaben, isobutylparaben
Ask Doctor Section
Discontinue use if signs of irritation or rash appear. If irritation or rash persists, consult a doctor
Warnings
For external use only. Not to be swallowed. Avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water
Keep out of reach of children