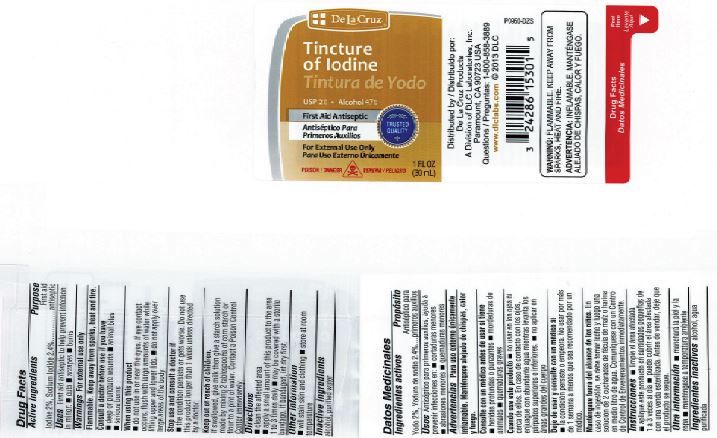
Active ingredient
Iodine 2%
Purpose
First Aid Antiseptic
Active ingredient
Sodium Iodide 2.4%
Purpose
First Aid Antiseptic
Active ingredient
Alcohol 47%
Purpose
First Aid Antiseptic
Indications
First aid to help prevent infection in minor cuts, scrapes and burns
Warnings
For external use only.
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
- Flammable: Keep away from sparks heat and flame
Stop use and consult doctor if
- the condition persists or gets worse, or if using for longer than one week
When using this product
- do not use in the eyes. If contact occurs, flush with large amounts of water while lifting upper and lower lids
- do not apply over large areas of the body
Keep out of reach of children.
In case of accidental ingestion, give milk then a starch solution made by mixing two tablespoonfuls of cornstarch or flour to a pint of water. Contact a Poison Control Center immediately.
Directions
- clean the affected area
- apply a small amount on the area 1 to 3 times daily
- may be covered with sterile bandage
- if bandaged let dry first
Other information
- will stain skin and clothing
Inactive ingredient
Purified Water
Label

DLC Laboratories, Inc.

