AMPROMED-P FOR POULTRY- amprolium powder
Bimeda, Inc., Division of Cross Vetpharm Group
----------
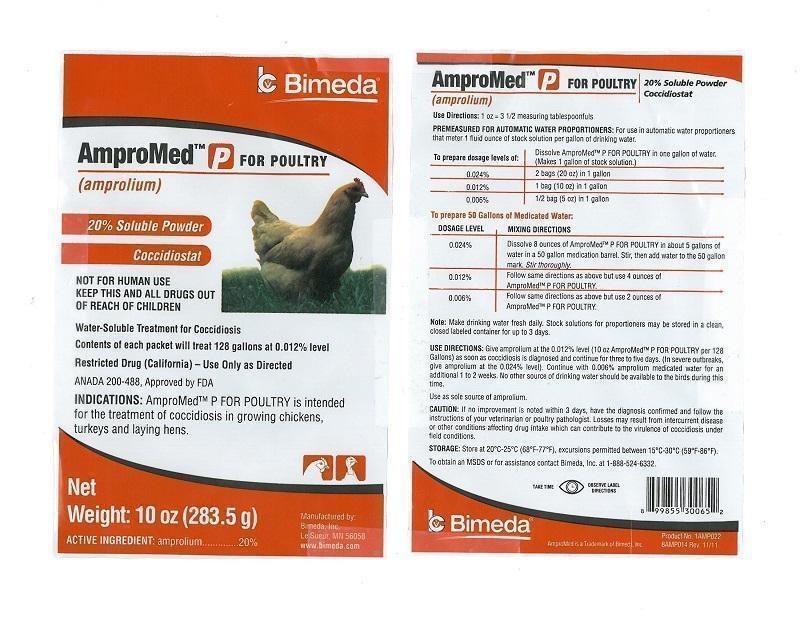
Use Directions: 1 oz = 3 1/2 measuring tablespoonfuls
PREMEASURED FOR AUTOMATIC WATER PROPORTIONERS: For use in automatic water proportioners that meter 1 fluid ounce of stock solution per gallon of drinking water.
To prepare dosage levels of: / Dissolve AmproMed P FOR POULTRY in one gallon of water. (Makes 1 gallon of stock solution.)
0.024% / 2 bags (20 oz) in 1 gallon
0.012% / 1 bag (10 oz) in 1 gallon
0.006% / 1/2 bag (5 oz) in 1 gallon
To prepare 50 Gallons of Medicated Water:
DOSAGE LEVEL / MIXING DIRECTIONS
0.024% / Dissolve 8 ounces of AmproMed P FOR POULTRY in about 5 gallons of water in a 50 gallon medication barrel. Stir, then add water to the 50 gallon mark. Stir thoroughly.
0.012% / Follow same directions as above but use 4 ounces of AmproMed P FOR POULTRY
0.006% / Follow same directions as above but use 2 ounces of AmproMed P FOR POULTRY
Note: Make drinking water fresh daily. Stock solutions for proportioners may be stored in a clean, closed labeled container for up to 3 days.
USE DIRECTIONS: Give amprolium at the 0.012% level (10 oz AmproMed P FOR POULTRY per 128 Gallons) as soon as coccidiosis is diagnosed and continue for three to five days. (In severe outbreaks, give amprolium at the 0.024% level). Continue with 0.006% amprolium medicated water for an additional 1 to 2 weeks. No other source of drinking water should be available to the birds during this time.
Use as sole source of amprolium.
CAUTION: If no improvement is noted within 3 days, have the diagnosis confirmed and follow the instructions of your veterinarian or poultry pathologist. Losses may result from intercurrent disease or other conditions affecting drug intake which can contribute to the virulence of coccidiosis under field conditions.
| AMPROMED-P FOR POULTRY
amprolium powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bimeda, Inc., Division of Cross Vetpharm Group (060492923) |
| Registrant - Bimeda, Inc., Division of Cross Vetpharm Group (060492923) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bimeda, Inc., Division of Cross Vetpharm Group | 060492923 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Juizhou | 654682061 | api manufacture | |