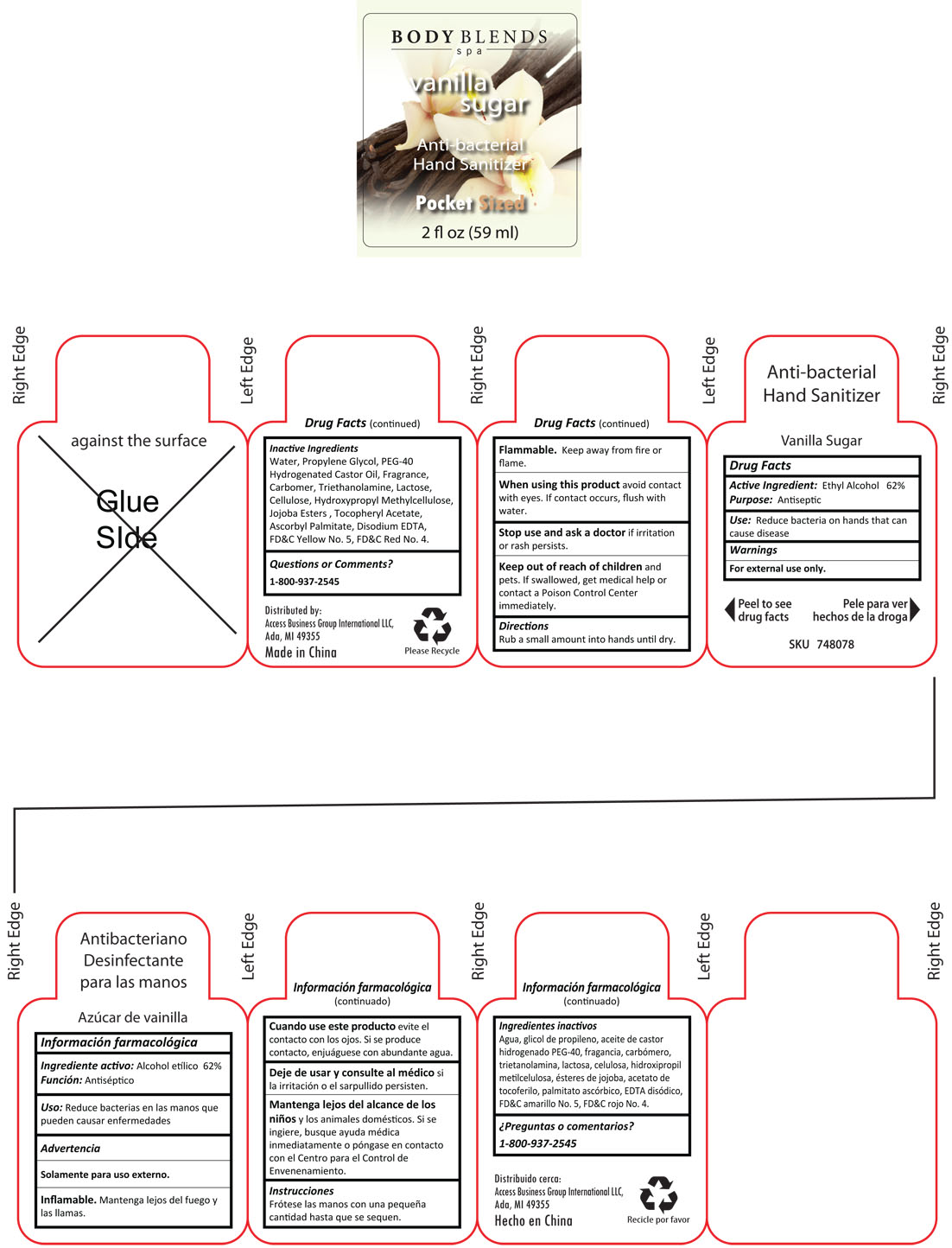
BODY BLENDS VANILLA SUGAR ANTI-BACTERIAL HAND SANITIZER - alcohol liquid
Xiamen Anna Global Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients Purpose
Ethyl Alcohol 62% Antiseptic
Uses Reduce bacteria on hands that can cause disease
Warnings
For external use only
Flammable: Keep away from fire or flame
When using this product,avoid
contact with eyes. If contact occurs, flush with water
Stop using and ask a doctor, if irritation or rash persists
Keep out of reach of children and pets. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Rub a small amount into hands until dry
Inactive ingredients
Water, Propylene Glycol, PEG-40 Hydrogenated Castor Oil, Fragrance, Carbomer, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Jojoba Ester, Tocopheryl Acetate, Ascorbyl Palmitate, Disodium EDTA, FD and C red No. 4, FD and C Yellow No. 5
lab