FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Ezetimibe tablets are indicated:
- In combination with a statin, or alone when additional low-density lipoprotein cholesterol (LDL-C) lowering therapy is not possible, as an adjunct to diet to reduce elevated LDL-C in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH).
- In combination with a statin as an adjunct to diet to reduce elevated LDL-C in pediatric patients 10 years of age and older with HeFH.
- In combination with fenofibrate as an adjunct to diet to reduce elevated LDL-C in adults with mixed hyperlipidemia.
- In combination with a statin, and other LDL-C lowering therapies, to reduce elevated LDL-C levels in adults and in pediatric patients 10 years of age and older with homozygous familial hypercholesterolemia (HoFH).
- As an adjunct to diet for the reduction of elevated sitosterol and campesterol levels in adults and in pediatric patients 9 years of age and older with homozygous familial sitosterolemia.
When ezetimibe tablets are used in combination with a statin, fenofibrate, or other LDL-C lowering therapies, refer to the Prescribing Information of these products for information on the safe and effective use.
2 DOSAGE AND ADMINISTRATION
- The recommended dose of ezetimibe tablet is 10 mg orally once daily, administered with or without food.
- If as dose is missed, take the missed dose as soon as possible. Do not double the next dose.
- Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating ezetimibe tablets.
- Administer ezetimibe tablets at least 2 hours before or 4 hours after administration of a bile acid sequestrant [ see Drug Interactions (7)].
3 DOSAGE FORMS AND STRENGTHS
10 mg tablets are white to off-white, capsule-shaped uncoated tablets with " E 10" debossed on one side and plain on other side.
4 CONTRAINDICATIONS
Ezetimibe tablets are contraindicated in patients with a known hypersensitivity to ezetimibe or any of the excipients in ezetimibe tablets. Hypersensitivity reactions including anaphylaxis, angioedema, rash, and urticaria have been reported [ see Adverse Reactions (6.2)].
When used in combination with a statin, fenofibrate, or other LDL-C lowering therapy, ezetimibe tablets are contraindicated in patients for whom a statin, fenofibrate, or other LDLC lowering therapy are contraindicated. Refer to the Prescribing Information of these products for a list of their contraindications [see Warnings and Precautions (5.1)]
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Combination Treatment with a Statin, Fenofibrate, or Other LDL-C Lowering Therapies
If ezetimibe tablets are administered with a statin, fenofibrate, or other LDL-C lowering therapies, refer to the Prescribing Information of these products for a description of their risks including, but not limited to, the warnings and precautions [see Contraindications (4)].
5.2 Liver Enzymes
Increases in serum transaminases have been reported with use of ezetimibe tablets [see Adverse Reactions (6.1)]. In controlled clinical combination studies of ezetimibe tablets initiated concurrently with a statin, the incidence of consecutive elevations (≥3 X ULN) in hepatic transaminase levels was 1.3% for patients treated with ezetimibe tablets administered with statins and 0.4% for patients treated with statins alone. Perform liver enzyme testing as clinically indicated and consider withdrawal of ezetimibe tablets if increases in ALT or AST ≥3 X ULN persist.
5.3 Myopathy/Rhabdomyolysis
Ezetimibe tablets may cause myopathy [muscle pain, tenderness, or weakness associated with elevated creatine kinase (CK)] and rhabdomyolysis [see Adverse Reactions (6.1)]. In post-marketing reports, most patients who developed rhabdomyolysis were taking a statin or other agents known to be associated with an increased risk of rhabdomyolysis, such as fibrates. If myopathy is suspected, discontinue ezetimibe tablets and other concomitant medications, as appropriate.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
• Liver enzyme abnormalities [see Warnings and Precautions (5.2)]
• Rhabdomyolysis and myopathy [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a
drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical
practice.
Monotherapy
In 10 double-blind, placebo-controlled clinical trials, 2,396 patients with primary hyperlipidemia (age range 9 to 86 years; 50% female, 90% White, 5% Black or African American, 2% Asian, 3% other races; 3% identified as Hispanic or Latino ethnicity) and elevated LDL-C were treated with ezetimibe tablets 10 mg daily for a median treatment duration of 12 weeks (range 0 to 39 weeks).
Adverse reactions reported in ≥ 2% of patients treated with ezetimibe tablets and at an incidence greater than placebo in placebo-controlled studies of ezetimibe tablets are shown in Table 1.
TABLE 1: Adverse Reactions Occurring in ≥ 2% and Greater than Placebo in ezetimibe tablets -treated Patients
| Adverse Reactions | Placebo (%) n=1,159 |
Ezetimibe tablets 10mg (%) n=2,396 |
| Upper respiratory tract infection | 2.5 | 4.3 |
| Diarrhea | 3.7 | 4.1 |
| Arthralgia | 2.2 | 3.0 |
| Sinusitis | 2.2 | 2.8 |
| Pain in extremity | 2.5 | 2.7 |
| Fatigue | 1.5 | 2.4 |
| 1.5 | 2.0 |
Combination with a Statin
In 28 double-blind, controlled (placebo or active-controlled) clinical trials, 11,308 patients with primary hyperlipidemia (age range 10 to 93 years, 48% female, 85% White, 7% Black or African
American, 3% Asian, 5% other races; 4% identified as Hispanic or Latino ethnicity) and elevated LDL-C were treated with ezetimibe tablets 10 mg/day concurrently with or added to on-going statin therapy for a median treatment duration of 8 weeks (range 0 to 112 weeks).
The incidence of consecutive increased transaminases (≥ 3 times ULN) was higher in patients receiving ezetimibe tablets administered with statins (1.3%) than in patients treated with statins alone (0.4%).
Adverse reactions reported in ≥2% of patients treated with ezetimibe tablets + statin and at an incidence greater than statin are shown in Table 2.
TABLE 2: Adverse Reactions Occurring ≥2% in ezetimibe tablets -treated Patients Co-administered with a Statin and at an Incidence Greater than Statin
| Adverse Reaction
All Statins* (%) n = 9,361 |
All Statins* (%) n = 9,361 |
Ezetimibe Tablets + All Statins* (%) n = 11,308 |
| Nasopharyngitis | 3.3 | 3.7 |
| Myalgia | 2.7 | 3.2 |
| Upper respiratory tract infection | 2.8 | 2.9 |
| Arthralgia | 2.4 | 2.6 |
| Diarrhea | 2.2 | 2.5 |
| Back pain | 2.3 | 2.4 |
| Influenza | 2.1 | 2.2 |
| Pain in extremity | 1.9 | 2.1 |
| Fatigue | 1.6 | 2.0 |
*All Statins = all doses of all statins
Combination with Fenofibrate
This clinical trial involving 625 patients with mixed dyslipidemia (age range 20 to 76 years; 44% female, 79% White, 1% Black or African American, 20% other races; 11% identified as Hispanic or Latino ethnicity) treated for up to 12 weeks and 576 patients treated for up to an additional 48 weeks evaluated co-administration of ezetimibe tablets and fenofibrate. Incidence rates for clinically important elevations (≥3 X ULN, consecutive) in hepatic transaminase levels were 4.5% and 2.7% for fenofibrate monotherapy (n=188) and ezetimibe tablets co-administered with fenofibrate (n=183), respectively, adjusted for treatment exposure. Corresponding incidence rates for cholecystectomy were 0.6% and 1.7% for fenofibrate monotherapy and ezetimibe tablets co-administered with fenofibrate, respectively [see Drug Interactions (7)].
6.2 Post-Marketing Experience
Because the reactions below are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following additional adverse reactions have been identified during post-approval use of ezetimibe tablets:
Blood Disorders:thrombocytopenia
Gastrointestinal Disorders:abdominal pain; pancreatitis; nausea
Hepatobiliary Disorders:elevations in liver transaminases, including elevations more than 5 X ULN; hepatitis; cholelithiasis; cholecystitis
Immune System Disorders:Hypersensitivity reactions including: anaphylaxis, angioedema, rash, and urticaria
Musculoskeletal Disorders:elevated creatine phosphokinase; myopathy/rhabdomyolysis
Nervous System Disorders:dizziness; paresthesia; depression; headache
Skin and Subcutaneous Tissue Disorders:erythema multiforme
7 DRUG INTERACTIONS
Table 3 includes a list of drugs with clinically important drug interactions when administered concomitantly with ezetimibe and instructions for preventing or managing them.
| Cyclosporine | |
| Clinical Impact | Concomitant use of ezetimibe tablets and cyclosporine increases ezetimibe and cyclosporine concentrations. The degree of increase in ezetimibe exposure may be greater in patients with severe renal insufficiency [see Clinical Pharmacology ( 12.3)] . |
| Intervention | Monitor cyclosporine concentrations in patients receiving ezetimibe tablets and cyclosporine. In patients treated with cyclosporine, weigh the potential effects of the increased exposure to ezetimibe from concomitant use against the benefits of alterations in lipid levels provided by ezetimibe tablets. |
| Fibrates | |
| Clinical Impact | Both fenofibrate and ezetimibe may increase cholesterol excretion into the bile, leading to cholelithiasis. Co-administration of ezetimibe tablets with fibrates other than fenofibrate is not recommended [see Adverse Reactions ( 6.1)] . |
| Intervention | If cholelithiasis is suspected in a patient receiving ezetimibe tablets and fenofibrate, gallbladder studies are indicated, and alternative lipid-lowering therapy should be considered. |
| Bile Acid Sequestrants | |
| Clinical Impact | Concomitant cholestyramine administration decreased the mean exposure of total ezetimibe. This may result in a reduction of efficacy [see Clinical Pharmacology ( 12.3)] . |
| Intervention | In patients taking a bile acid sequestrant, administer ezetimibe tablets at least 2 hours before or 4 hours after the bile acid sequestrant [see Dosage and Administration ( 2)] . |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient data on ezetimibe use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, no adverse developmental effects were observed in pregnant rats and rabbits orally administered ezetimibe during the period of organogenesis at doses that resulted in up to 10 and 150 times, respectively, the human exposure at the MRHD, based on AUC (see Data). Ezetimibe tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. When ezetimibe tablets are administered with a statin, refer to the Prescribing Information for the statin.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In oral (gavage) embryo-fetal development studies of ezetimibe conducted in rats (gestation days 6-15) and rabbits (gestation days 7-19), there was no evidence of maternal toxicity or embryolethal effects at the doses tested (250, 500, 1,000 mg/kg/day). In rats, increased incidences of common fetal skeletal findings (extra pair of thoracic ribs, unossified cervical vertebral centra, shortened ribs) were observed at 1,000 mg/kg/day (~10 times the human exposure at 10 mg daily based on AUC 0-24hrfor total ezetimibe). In rabbits treated with ezetimibe, an increased incidence of extra thoracic ribs was observed at 1,000 mg/kg/day (150 times the human exposure at 10 mg daily based on AUC 0-24hrfor total ezetimibe). The animal-to-human exposure multiple for total ezetimibe at the no-observed effect level was 6 times for rat and 134 times for rabbit. Fetal exposure to ezetimibe (conjugated and unconjugated) was confirmed in subsequent placental transfer studies conducted using a maternal dose of 1,000 mg/kg/day. The fetal maternal plasma exposure ratio (total ezetimibe) was 1.5 for rats on gestation day 20 and 0.03 for rabbits on gestation day 22.
The effect of ezetimibe on prenatal and postnatal development and maternal function was evaluated in pregnant rats at doses of 100, 300 or 1,000 mg/kg/day from gestation day 6 through lactation day 21. No maternal toxicity or adverse developmental outcomes were observed up to and including the highest dose tested (17 times the human exposure at 10 mg daily based on AUC 0-24hrfor total ezetimibe).
Multiple-dose studies of ezetimibe given in combination with statins in rats and rabbits during organogenesis resulted in higher ezetimibe and statin exposures. Reproductive findings occurred at lower doses in combination therapy compared to monotherapy.
8.2 Lactation
Risk Summary
There is no information about the presence of ezetimibe in human milk. Ezetimibe is present in rat milk (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. There is no information about the effects of ezetimibe on the breastfed infant or the effects of ezetimibe on milk production. Ezetimibe tablets should not be used in nursing mothers unless the potential benefit justifies the potential risk to the infant.
Data
Ezetimibe was present in the milk of lactating rats. The pup to maternal plasma ratio for total ezetimibe was 0.5 on lactation day 12.
8.4 Pediatric Use
The safety and effectiveness of ezetimibe tablets in combination with a statin as an adjunct to diet to reduce LDL-C have been established in pediatric patients 10 years of age and older with HeFH. Use of ezetimibe tablets for this indication is based on a double-blind, placebo-controlled clinical trial in 248 pediatric patients (142 males and 106 postmenarchal females) 10 years of age and older with HeFH [see Clinical Studies (14)]. In this limited controlled trial, there was no significant effect on growth or sexual maturation in the adolescent males or females, or on menstrual cycle length in females.
The safety and effectiveness of ezetimibe tablets in combination with a statin, and other LDL-C lowering therapies, to reduce LDL-C have been established in pediatric patients 10 years of age and older with HoFH. Use of ezetimibe tablets for this indication is based on a 12-week double-blind, placebo-controlled clinical trial followed by an uncontrolled extension period in 7 pediatric patients 11 years of age and older with HoFH [see Clinical Studies (14)].
The safety and effectiveness of ezetimibe tablets as an adjunct to diet for the reduction of elevated sitosterol and campesterol levels have been established in adults and pediatric patients 9 years of age and older with homozygous familial sitosterolemia. Use of ezetimibe tablets for this indication is based on an 8-week double-blind, placebo-controlled clinical trial in 4 patients 9 years of age and older with homozygous sitosterolemia with elevated plasma sitosterol levels (>5 mg/dL) [see Clinical Studies (14)].
The safety and effectiveness of ezetimibe tablets have not been established in pediatric patients younger than 10 years of age with HeFH or HoFH, in pediatric patients younger than 9 years of age with homozygous familial sitosterolemia, or in pediatric patients with other types of hyperlipidemia.
8.5 Geriatric Use
Of the 2,396 patients who received ezetimibe tablets in clinical trials, 669 (28%) were 65 years of age and older, and 111 (5%) were 75 years of age and older. Of the 11,308 patients who received ezetimibe tablets in combination with a statin in clinical trials, 3587 (32%) were 65 years of age and older, and 924 (8%) were 75 years of age and older [see Clinical Studies (14)]. No overall differences in safety or effectiveness of ezetimibe tablets have been observed between patients 65 years of age and older and younger patients. No clinically meaningful differences in the pharmacokinetics of ezetimibe were observed in geriatric patients compared to younger adult patients [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dosage adjustment of ezetimibe tablets is necessary in patients with renal impairment.
8.7 Hepatic Impairment
Ezetimibe tablets are not recommended for use in patients with moderate to severe hepatic impairment (Child-Pugh B or C) due to the unknown effects of the increased exposure to ezetimibe [ see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
In the event of overdose, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
11 DESCRIPTION
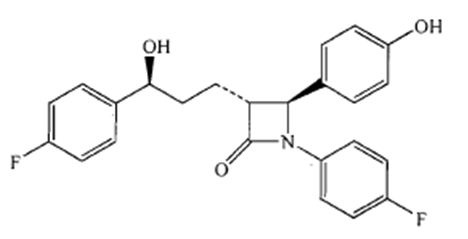
Ezetimibe is a dietary cholesterol absorption inhibitor. The chemical name of ezetimibe is 1-(4- fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone. The empirical formula is C24H21F2NO3. Its molecular weight is 409.42 and its structural formula is:
Ezetimibe is a white to off white, crystalline powder that is freely soluble in methanol, and acetone, soluble in ethanol, and practically insoluble in water. Ezetimibe has a melting point of about 165.13°C and is stable at ambient temperature. Ezetimibe is available as a tablet for oral administration containing 10 mg of ezetimibe and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone, pregelatinized starch (maize) and sodium lauryl sulfate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ezetimibe reduces blood cholesterol by inhibiting the absorption of cholesterol by the small intestine.
The molecular target of ezetimibe has been shown to be the sterol transporter,
Niemann-Pick C1-Like 1 (NPC1L1), which is involved in the intestinal uptake of cholesterol and
phytosterols. Ezetimibe localizes at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in LDL receptors, resulting in clearance of cholesterol from the blood.
12.2 Pharmacodynamics
Ezetimibe tablets reduces total cholesterol (total-C), LDL-C, apolipoprotein (Apo) B, and non-high-density lipoprotein cholesterol (non-HDL-C) in patients with hyperlipidemia.
In a 2-week clinical trial in 18 hypercholesterolemic patients, ezetimibe tablets inhibited intestinal cholesterol absorption by 54%, compared with placebo. Ezetimibe tablets had no clinically meaningful effect on the plasma concentrations of the fat-soluble vitamins A, D, and E (in a trial of 113 patients) and did not impair adrenocortical steroid hormone production (in a trial of 118 patients).
12.3 Pharmacokinetics
Absorption
After oral administration, ezetimibe is absorbed and extensively conjugated to a pharmacologically active phenolic glucuronide (ezetimibe-glucuronide). After a single 10 mg dose of ezetimibe tablets to fasted adults, mean ezetimibe peak plasma concentrations (C max) of 3.4 to 5.5 ng/mL were attained within 4 to 12 hours (T max). Ezetimibe-glucuronide mean C maxvalues of 45 to 71 ng/mL were achieved between 1 and 2 hours (T max). There was no substantial deviation from dose proportionality between 5 and 20 mg. The absolute bioavailability of ezetimibe cannot be determined, as the compound is virtually insoluble in aqueous media suitable for injection.
Effect of Food
Concomitant food administration (high-fat or non-fat meals) had no effect on the extent of absorption of ezetimibe when administered as ezetimibe 10 mg tablets. The C maxvalue of ezetimibe was increased by 38% with consumption of high-fat meals.
Distribution
Ezetimibe and ezetimibe-glucuronide are highly bound (> 90%) to human plasma proteins.
Elimination
Metabolism
Ezetimibe is primarily metabolized in the small intestine and liver via glucuronide conjugation (a phase II reaction) with subsequent biliary and renal excretion. Minimal oxidative metabolism (a phase I reaction) has been observed in all species evaluated. In humans, ezetimibe is rapidly metabolized to ezetimibe-glucuronide. Ezetimibe and ezetimibe-glucuronide are the major drug-derived compounds detected in plasma, constituting approximately 10 to 20% and 80 to 90% of the total drug in plasma, respectively.
Both ezetimibe and ezetimibe-glucuronide are eliminated from plasma with a half-life of approximately 22 hours for both ezetimibe and ezetimibe-glucuronide. Plasma concentration-time profiles exhibit multiple peaks, suggesting enterohepatic recycling
Excretion
Following oral administration of 14C-ezetimibe (20 mg) to human subjects, total ezetimibe (ezetimibe + ezetimibe-glucuronide) accounted for approximately 93% of the total radioactivity in plasma. After 48 hours, there were no detectable levels of radioactivity in the plasma.
Approximately 78% and 11% of the administered radioactivity were recovered in the feces and urine, respectively, over a 10-day collection period. Ezetimibe was the major component in feces and accounted for 69% of the administered dose, while ezetimibe-glucuronide was the major component in urine and accounted for 9% of the administered dose.
Specific Populations
Geriatric Patients: In a multiple-dose trial with ezetimibe given 10 mg once daily for 10 days, plasma concentrations for total ezetimibe were about 2-fold higher in older (≥ 65 years) healthy subjects compared to younger subjects. However, the difference in plasma concentrations is not clinically meaningful.
Gender: In a multiple-dose trial with ezetimibe given 10 mg once daily for 10 days, plasma concentrations for total ezetimibe were slightly higher (< 20%) in females than in males.
Race: Based on a meta-analysis of multiple-dose pharmacokinetic studies, there were no pharmacokinetic differences between Black and White subjects. Studies in Asian subjects indicated that the pharmacokinetics of ezetimibe were similar to those seen in White subjects.
Renal Impairment:After a single 10-mg dose of ezetimibe in patients with severe renal disease (n=8; mean CrCl ≤30 mL/min/1.73 m 2), the mean AUC values for total ezetimibe, ezetimibe-glucuronide, and ezetimibe were increased approximately 1.5-fold, compared to healthy subjects (n=9).
Hepatic Impairment:After a single 10-mg dose of ezetimibe, the mean AUC for total ezetimibe was increased approximately 1.7-fold in patients with mild hepatic impairment (Child-Pugh score 5 to 6), compared to healthy subjects. The mean AUC values for total ezetimibe and ezetimibe were increased approximately 3-to 4-fold and 5-to 6-fold, respectively, in patients with moderate (Child-Pugh score 7 to 9) or severe hepatic impairment (Child-Pugh score 10 to 15). In a 14-day, multiple-dose trial (10 mg daily) in patients with moderate hepatic impairment, the mean AUC values for total ezetimibe and ezetimibe were increased approximately 4-fold on Day 1 and Day 14 compared to healthy subjects [see Use in Specific Populations (8.7)].
Drug Interactions
Ezetimibe tablets had no significant effect on a series of probe drugs (caffeine, dextromethorphan, tolbutamide, and IV midazolam) known to be metabolized by cytochrome P450 (1A2, 2D6, 2C8/9 and 3A4) in a “cocktail” trial of twelve healthy adult males. This indicates that ezetimibe is neither an inhibitor nor an inducer of these cytochrome P450 isozymes, and it is unlikely that ezetimibe will affect the metabolism of drugs that are metabolized by these enzymes.
TABLE 4: Effect of Coadministered Drugs on Total Ezetimibe
| Coadministered Drug and Dosing Regimen | Total Ezetimibe* | |
| Change in AUC | Change in C max | |
| Cyclosporine-stable dose required (75 mg to 150 mg BID )†, ‡ | ↑240% | ↑290% |
| Fenofibrate, 200 mg QD, 14 days ‡ | ↑48% | ↑64% |
| Gemfibrozil, 600 mg BID, 7 days ‡ | ↑64% | ↑91% |
| Cholestyramine, 4 g BID, 14 days ‡ | ↓55% | ↓4% |
| Aluminum & magnesium hydroxide combination antacid, single dose § | ↓4% | ↓30% |
| Cimetidine, 400 mg BID, 7 days | ↑6% | ↑22% |
| Glipizide, 10 mg, single dose | ↑4% | ↓8% |
| Statins | ||
| Lovastatin 20 mg QD, 7 days | ↑9% | ↑3% |
| Pravastatin 20 mg QD, 14 days | ↑7% | ↑23% |
| Atorvastatin 10 mg QD, 14 days | ↓2% | ↑12% |
| Rosuvastatin 10 mg QD, 14 days | ↑13% | ↑18% |
| Fluvastatin 20 mg QD, 14 days | ↓19% | ↑7% |
* Based on 10 mg dose of ezetimibe
† Post-renal transplant patients with mild impaired or normal renal function. In a different trial, a renal transplant patient with severe renal insufficiency (creatinine clearance of 13.2 mL/min/1.73 m2) who was receiving multiple medications, including cyclosporine, demonstrated a 12-fold greater exposure to total ezetimibe compared to healthy subjects.
‡ See Drug Interactions (7).
§ Supralox, 20 mL
TABLE 5: Effect of Ezetimibe Co-Administration on Systemic Exposure to Other Drugs
|
Coadministered Drug and its Dosage Regimen | Ezetimibe Dosage Regimen | Change in AUC of Coadministered Drug | Change in C max of
Coadministered Drug |
| Warfarin, 25 mg single dose on day 7 | 10 mg QD, 11 days | ↓2% (R-warfarin)
↓4% (S-warfarin) | ↑3% (R-warfarin)
↑1% (S-warfarin) |
| Digoxin, 0.5 mg single dose | 10 mg QD, 8 days | ↑2% | ↓7% |
| Gemfibrozil, 600 mg BID, 7 days* | 10 mg QD, 7 days | ↓1% | ↓11% |
| Ethinyl estradiol & Levonorgestrel, QD, 21 days | 10 mg QD, days 8 to 14 of 21d oral contraceptive cycle | Ethinyl estradiol 0% Levonorgestrel
0% | Ethinyl estradiol
↓9% Levonorgestrel ↓5% |
| Glipizide, 10 mg on days 1 and 9 | 10 mg QD, days 2 to 9 | ↓3% | ↓5% |
| Fenofibrate, 200 mg QD, 14 days* | 10 mg QD, 14 days | ↑11% | ↑7% |
| Cyclosporine, 100 mg single dose day 7* | 20 mg QD, 8 days | ↑15% | ↑10% |
| Statins | |||
| Lovastatin 20 mg QD, 7 days | 10 mg QD, 7 days | ↑19% | ↑3% |
| Pravastatin 20 mg QD, 14 days | 10 mg QD, 14 days | ↓20% | ↓24% |
| Atorvastatin 10 mg QD, 14 days | 10 mg QD, 14 days | ↓4% | ↑7% |
| Rosuvastatin 10 mg QD, 14 days | 10 mg QD, 14 days | ↑19% | ↑17% |
| Fluvastatin 20 mg QD, 14 days | 10 mg QD, 14 days | ↓39% | ↓27% |
* See Drug Interactions (7).
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 104-week dietary carcinogenicity study with ezetimibe was conducted in rats at doses up to 1500 mg/kg/day (males) and 500 mg/kg/day (females) (~20 times the human exposure at 10 mg daily based on AUC 0-24hrfor total ezetimibe). A 104-week dietary carcinogenicity study with ezetimibe was also conducted in mice at doses up to 500 mg/kg/day (> 150 times the human exposure at 10 mg daily based on AUC 0-24hrfor total ezetimibe). There were no statistically significant increases in tumor incidences in drug-treated rats or mice.
No evidence of mutagenicity was observed in vitroin a microbial mutagenicity (Ames) test with Salmonella typhimuriumand Escherichia coliwith or without metabolic activation. No evidence of clastogenicity was observed in vitroin a chromosomal aberration assay in human peripheral blood lymphocytes with or without metabolic activation. In addition, there was no evidence of genotoxicity in the in vivomouse micronucleus test.
In oral (gavage) fertility studies of ezetimibe conducted in rats, there was no evidence of reproductive toxicity at doses up to 1000 mg/kg/day in male or female rats (~7 times the human exposure at 10 mg daily based on AUC 0-24hrfor total ezetimibe).
16 HOW SUPPLIED/STORAGE AND HANDLING
Ezetimibe tablets, USP, 10 mg, are white to off-white, capsule-shaped uncoated tablets with “ E 10” debossed on one side and plain on other side. They are supplied as follows:
NDC51660-200-30 Bottles of 30
NDC51660-200-90 Bottles of 90
NDC51660-200-05 Bottles of 500
Storage
Store at 20° - 25° C (68° - 77° F) [See USP Controlled Room Temperature]. Protect from moisture.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-Approved Patient Labeling (Patient Information).
Inform patients that ezetimibe tablets may cause liver enzyme elevations [see Warnings and Precautions (5.2)].
Muscle Pain
Advise patients that ezetimibe tablets may cause myopathy and rhabdomyolysis. Inform patients that the risk is also increased when taking certain types of medication and they should discuss all medication, both prescription and over the counter, with their healthcare provider. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever [see Warnings and Precautions (5.3), andDrug Interactions (7)].
Pregnancy
Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if ezetimibe tablets should be discontinued [see Use in Specific Populations (8.1)].
Breastfeeding
Advise patients who have a lipid disorder and are breastfeeding to discuss the options with their healthcare provider [see Use in Specific Populations(8.2)].
Missed Dose
Instruct patients to take ezetimibe tablets only as prescribed. If a dose is missed, it should be taken as soon as possible. Advise patients not to double their next dose.
Manufactured by:
Ohm Laboratories Inc.
New Brunswick, NJ 08901
Distributed by:
Sun Pharmaceuticals Industries, Inc.
Cranbury, NJ 08512
March 2024 FDA-07
5249048
Patient Information
EZETIMIBE TABLETS, USP
(e-zet’-i-mibe)
Rx only
Read this information carefully before you start taking ezetimibe tablets, USP and each time you get more ezetimibe tablets, USP. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about ezetimibe tablets, USP, ask your doctor. Only your doctor can determine if ezetimibe tablets, USP are right for you.
What are ezetimibe tablets, USP?
Ezetimibe tablets, USP are used with a cholesterol lowering diet:
- and with other cholesterol medicines called a statin, or alone (when additional cholesterol lowering treatments are not possible), to lower elevated low-density lipoprotein cholesterol (LDL-C) or bad cholesterol in adults with primary hyperlipidemia (too many fats in your blood), including heterozygous familial hypercholesterolemia (HeFH). HeFH is an inherited condition that causes high levels of bad cholesterol.
- and with a statin to lower LDL-C in adults and children 10 years of age and older with HeFH.
- and with a medicine called fenofibrate to lower elevated LDL-C in adults with mixed hyperlipidemia.
to lower elevated sitosterol and campesterol levels in adults and in children 9 years of age and older with homozygous familial sitosterolemia (a rare inherited condition that prevents the body from getting rid of cholesterol from plants).
Ezetimibe tablets is also used:
- with a statin and other cholesterol lowering treatments to lower elevated LDL-C levels in adults and patients 10 years of age and older with homozygous familial hypercholesterolemia (HoFH). HoFH is an inherited condition that causes high levels of bad cholesterol.
The safety and effectiveness of ezetimibe tablets has not been established in children:
- younger than 10 years of age with HeFH or HoFH.
- younger than 9 years of age with homozygous familial sitosterolemia.
- with other types of hyperlipemia.
Do not take ezetimibe tablets:
- if you are allergic to ezetimibe or any of the ingredients in ezetimibe tablets. See the end of this Patient Information leaflet for a complete list of ingredients in ezetimibe tablets. Stop using ezetimibe tablets and get medical help right away if you have symptoms of a serious allergic reaction including:
o swelling of the face, tongue, or throat
o difficulty breathing or swallowing
o fainting or feeling dizzy o very fast heartbeat
o severe skin rash, hives, and itching
o flu-like symptoms including fever, sore throat, cough, tiredness, and joint pain
- with certain statins, fenofibrate, or other LDL-C lowering medicines if your healthcare provider has told you not to take them.
Before you take ezetimibe tablets, tell your healthcare provider about all your medical conditions, including if you:
- have liver problems. Ezetimibe tablets may not be right for you.
- are pregnant or plan to become pregnant. It is not known if ezetimibe tablets will harm your unborn baby. You and your healthcare provider should decide if you will take ezetimibe tablets while you are pregnant.
- are breastfeeding. It is not known if ezetimibe tablets passes into your breast milk. You and your healthcare provider should decide the best way to feed your baby if you take ezetimibe tablets.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. Talk to your healthcare provider before you start taking any new medicines. Taking ezetimibe tablets with certain other medicines may affect each other causing side effects. Ezetimibe tablets may affect the way other medicines work, and other medicines may affect how ezetimibe tablets works.
Especially tell your healthcare provider if you take:
- cyclosporine (a medicine for your immune system)
- fibrates (medicine for lowering cholesterol)
- bile acid sequestrants (medicine for lowering LDL-C)
Ask your healthcare provider or pharmacist for a list of medicines if you are not sure. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take ezetimibe tablets?
- Take ezetimibe tablets 1-time each day, with or without food. It may be easier to remember to take your dose if you do it at the same time every day, such as with breakfast, dinner, or at bedtime. If you also take another medicine to reduce your cholesterol, ask your healthcare provider if you can take them at the same time.
- If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take 2 doses of ezetimibe tablets at the same time.
- While taking ezetimibe tablets, continue to follow your cholesterol-lowering diet and to exercise as your healthcare provider told you to.
- If you take a medicine called a bile acid sequestrant, take ezetimibe tablets at least 2 hours before or 4 hours after you take the bile acid sequestrant.
- Your healthcare provider may do blood tests to check your LDL-C levels as early as 4 weeks after starting treatment with ezetimibe tablets.
- In case of an overdose, get medical help or contact a live Poison Center expert right away at 1-800-222-1222. Advice is also available online at poisonhelp.org.
What are the possible side effects of ezetimibe tablets?
Ezetimibe tablets may cause serious side effects including:
- increased liver enzymes.An increase in liver enzymes can happen in people taking ezetimibe tablets alone or with statins. Your healthcare provider may do blood tests to check your liver before and during treatment. Your healthcare provider may need to change or stop your treatment with ezetimibe tablets because of an increase in liver enzymes.
- muscle pain, tenderness, and weakness (myopathy). Muscle problems, including muscle breakdown (rhabdomyolysis) can happen. Tell your healthcare provider right away if:
- you have unexplained muscle pain, tenderness, weakness, feel more tired than usual, or fever.
- you have muscle problems that do not go away even after your healthcare provider has advised you to stop taking ezetimibe tablets. Your healthcare provider may do further tests to diagnose the cause of your muscle problems.
Your chances of getting muscle problems are higher if you are also taking statins or fibrates.
The most common side effects of ezetimibe tablets taken alone include:
- upper respiratory
- joint pain
- pain in arms or
- flu-like tract infection legs symptoms
- diarrhea
- inflammation of the
- feeling tired sinuses
The most common side effects of ezetimibe tablets taken with a statin include:
- runny nose, sore throat
- joint pain
- flu-like symptoms
- muscle aches and pains
- diarrhea
- pain in arms or legs
- upper respiratory tract infection
- back pain
- feeling tired
Tell your healthcare provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of ezetimibe tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ezetimibe tablets?
- Store ezetimibe tablets at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Protect from moisture. Keep ezetimibe tablets and all medicines out of the reach of children.
General information about safe and effective use of ezetimibe tablets.Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ezetimibe tablets for a condition for which it was not prescribed. Do not give ezetimibe tablets to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ezetimibe tablets that is written for health professionals.
What are the ingredients in Ezetimibe tablets?
Active ingredient:ezetimibe.
Inactive ingredients:Croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone, pregelatinized starch (maize) and sodium lauryl sulfate.
Manufactured by:
Ohm Laboratories Inc.
New Brunswick, NJ 08901
Distributed by:
Sun Pharmaceuticals Industries, Inc.
Cranbury, NJ 08512
March 2024 FDA-07