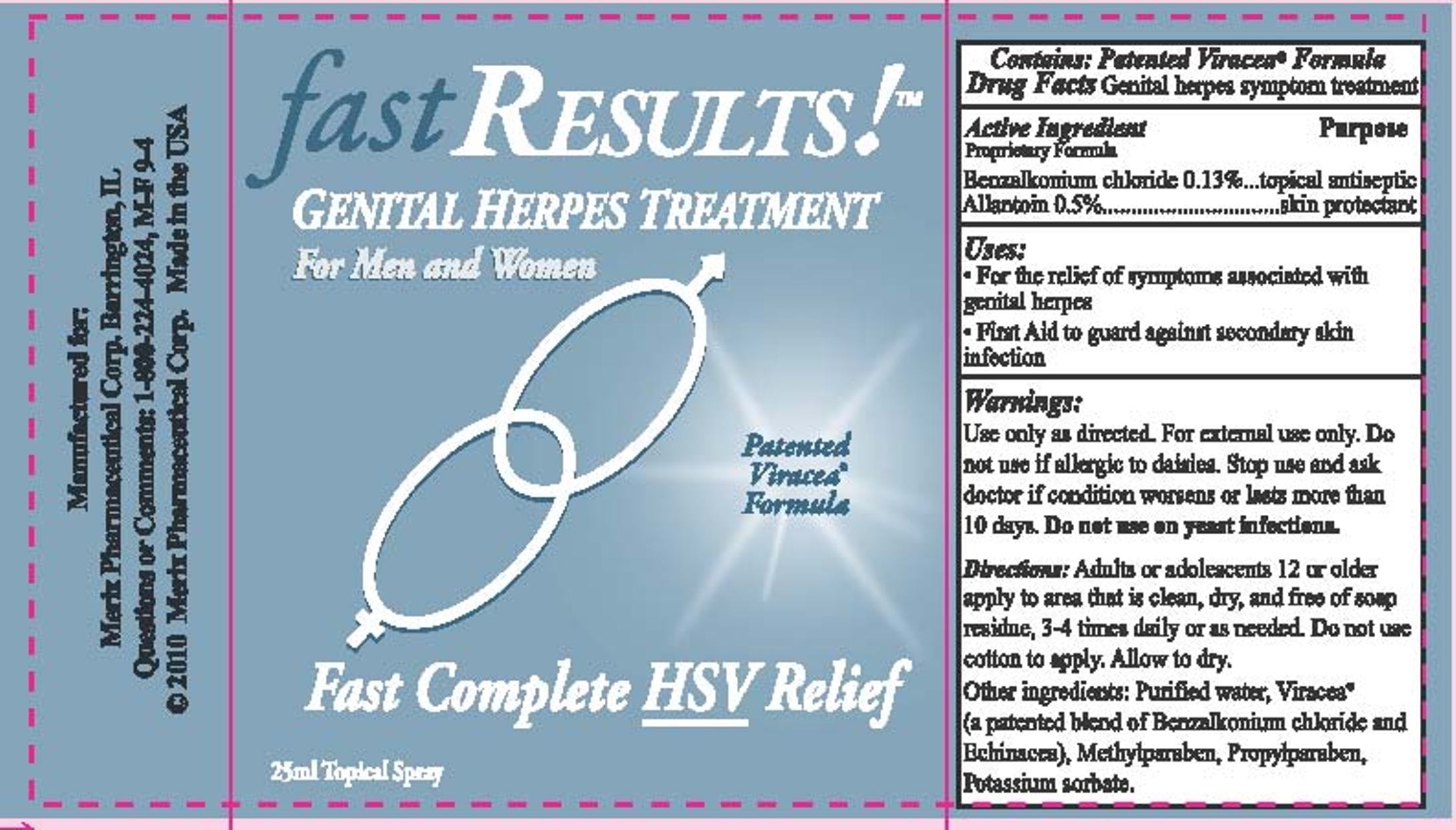
USES:
For the relief of symptoms associated with genital herpesFirst Aid to guard against secondary skin infection
WARNINGS:
Use only as directed. For external use only. Do not use if allergic to daisies.Stop use and ask doctor if condition worsens or last more than 10 days.
Do not use on yeast infections.
KEEP OUT OF REACH OF CHILDREN:
Keep this and all drugs out of reach of children.
In case of accidental ingestion other then intended use, seek professional assistance
or call a poison control center.
DIRECTIONS:
Adults or adolescents 12 or older apply to clean dry area free of soap or cleanser residue.