NIGHTTIME SLEEP AID- doxylamine succinate tablet
Wal-Mart Stores Inc
----------
Equate 44-386-Bisect-Delisted
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as asthma, emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Directions
- adults: take one tablet 30 minutes before going to bed; take once daily or as directed by a doctor
- do not give to children under 12 years of age
Other information
- store at controlled room temperature 20º-25ºC (68º-77ºF)
- use by expiration date on package
Inactive ingredients
dicalcium phosphate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
Principal display panel
NDC 49035-863-89
equate™
Compare to Unisom® SleepTabs® active Ingredient*
Sleep Aid
Doxylamine Succinate, 25 mg
Nighttime Sleep-Aid
One tablet per dose
• Easy to swallow
Actual Size
32 TABLETS
DISTRIBUTED BY: Walmart Inc., Bentonville, AR 72716
PRODUCT OF ITALY
*This product is not manufactured or distributed by Chattem, Inc., owner of the registered trademark Unisom® SleepTabs®.
50844 REV0513A38627
729665
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Equate 44-386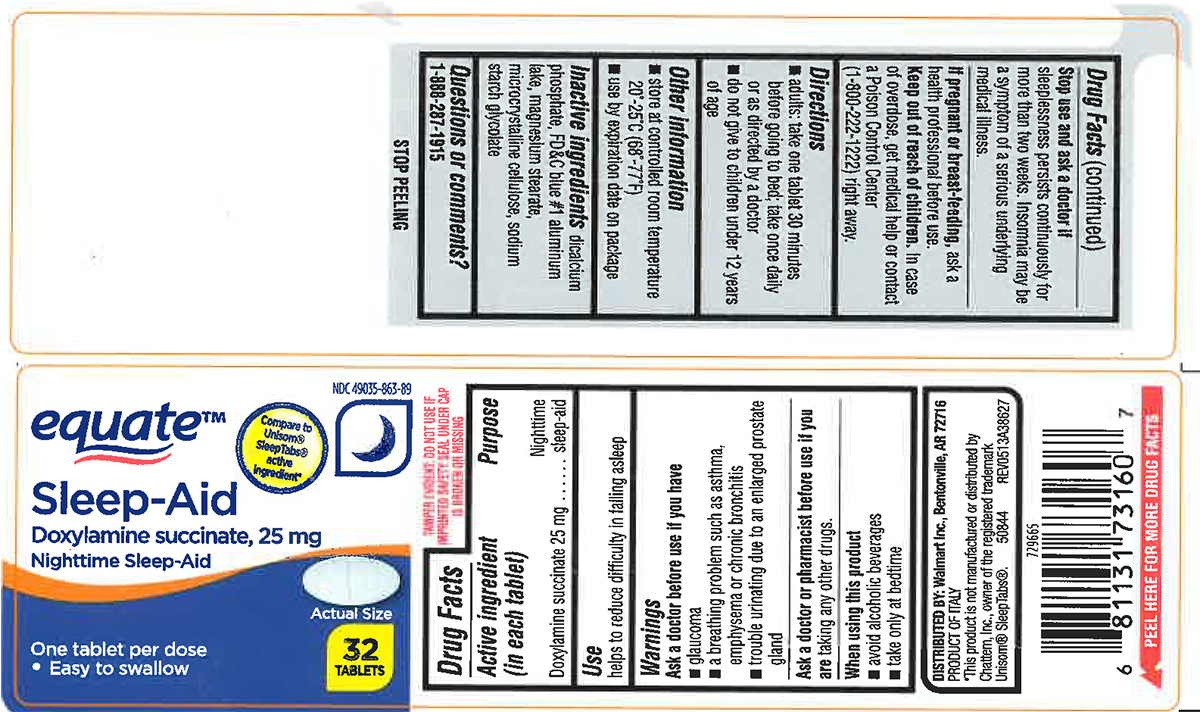
| NIGHTTIME SLEEP AID
doxylamine succinate tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Wal-Mart Stores Inc (051957769) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(49035-863) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(49035-863) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | PACK(49035-863) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(49035-863) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | PACK(49035-863) | |
Revised: 11/2019
Document Id: 92e2c9e3-169a-431f-9587-147852a96015
Set id: 5b0bf558-31e9-4b95-b946-84d160a73572
Version: 10
Effective Time: 20191113
Wal-Mart Stores Inc