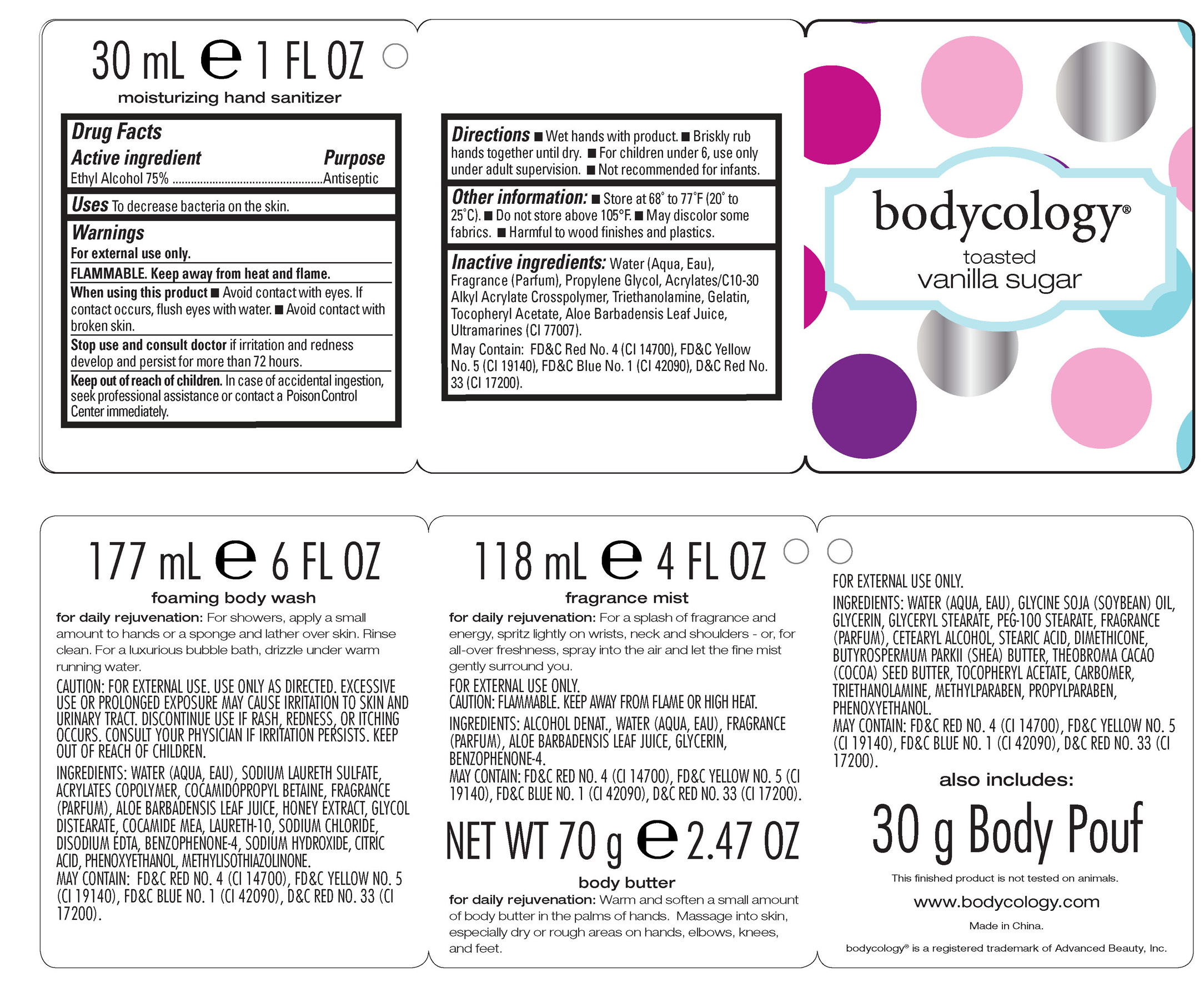
Active Ingredient purpose
Ethyl Alcohol 75% Antiseptic
Warnings: For external use only.
Flammable. Keep away from heat and flame.
When using this product: Avoid contact with eyes. If contact occurs, flush eyes with water. Avoid contact with broken skin.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Flammable. Keep away from heat and flame.
When using this product: Avoid contact with eyes. If contact occurs, flush eyes with water. Avoid contact with broken skin.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions: Wet hands with product.
Briskly rub hands together until dry.
For children under 6, use only under adult supervision.
Not recommended for infants.
Inactive ingredients: Water (aqua, eau), fragrance (parfum), Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer
triethanolamine, Gelatin, Tocopheryl Acetate, Aloe Barbadensis Leaf Juice, Ultramarines (CI77007)
May contain: FD C Red No.4(CI 14700), FD C Yellow No. 5 (CI 19140), FD C Blue No.1 (CI 42090), D C Red No 33(CI 17200)