Warnings
For occasional use only
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
Directions
- adults and children 12 years and over: take 1 tablet not more often than every 3 to 4 hours
- children under 12 years: do not use
Other information
- each tablet contains: calcium 35 mg
-
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, dextrates hydrated, dibasic calcium phosphate dihydrate, FD&C yellow #6 aluminum lake, magnesium stearate, microcrystalline cellulose, silicon dioxide
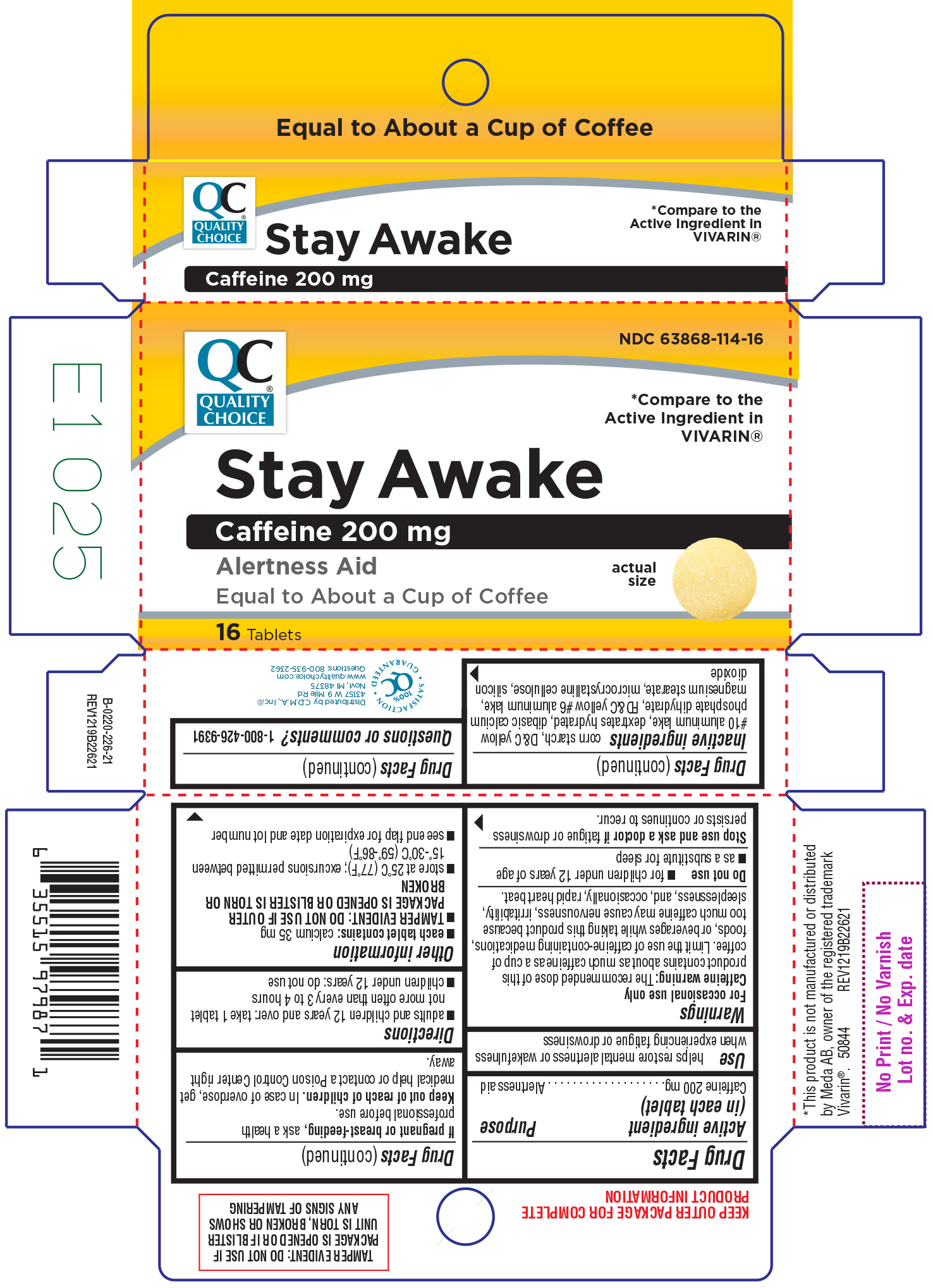
Principal Display Panel
NDC 63868-114-16
QC®
QUALITY
CHOICE
*Compare to the
Active ingredient in
VIVARIN®
Stay Awake
Caffeine 200 mg
Alertness Aid
Equal to About a Cup of Coffee
actual
size
16 Tablets
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed
by Meda AB, owner of the registered trademark
Vivarin®. 50844 REV1219B22621
SATISFACTION
GUARANTEED
100%
QC
Distributed by C.D.M.A., Inc©
43157 W 9 Mile Rd
Novi, MI 48375
www.qualitychoice.com
Questions:800-935-2362
Quality Choice 44-226