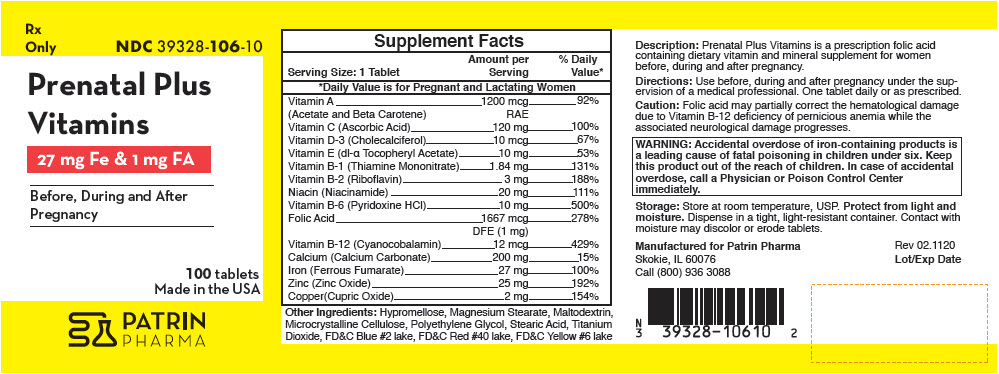
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Tablet | Amount per Serving | % Daily Value* |
|
||
| Vitamin A | 1200 mcg | 92% |
| (Acetate and Beta Carotene) | RAE | |
| Vitamin C (Ascorbic Acid) | 120 mg | 100% |
| Vitamin D-3 (Cholecalciferol) | 10 mcg | 67% |
| Vitamin E (dl-α Tocopheryl Acetate) | 10 mg | 53% |
| Vitamin B-1 (Thiamine Mononitrate) | 1.84 mg | 131% |
| Vitamin B-2 (Riboflavin) | 3 mg | 188% |
| Niacin (Niacinamide) | 20 mg | 111% |
| Vitamin B-6 (Pyridoxine HCl) | 10 mg | 500% |
| Folic Acid | 1667 mcg | 278% |
| DFE (1 mg) | ||
| Vitamin B-12 (Cyanocobalamin) | 12 mcg | 429% |
| Calcium (Calcium Carbonate) | 200 mg | 15% |
| Iron (Ferrous Fumarate) | 27 mg | 100% |
| Zinc (Zinc Oxide) | 25 mg | 192% |
| Copper(Cupric Oxide) | 2 mg | 154% |
Other Ingredients: Hypromellose, Magnesium Stearate, Maltodextrin, Microcrystalline Cellulose, Polyethylene Glycol, Stearic Acid, Titanium Dioxide, FD&C Blue #2 lake, FD&C Red #40 lake, FD&C Yellow #6 lake
Description
Prenatal Plus Vitamins is a prescription folic acid containing dietary vitamin and mineral supplement for women before, during and after pregnancy.
Directions
Use before, during and after pregnancy under the supervision of a medical professional. One tablet daily or as prescribed.
Caution
Folic acid may partially correct the hematological damage due to Vitamin B-12 deficiency of pernicious anemia while the associated neurological damage progresses.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under six. Keep this product out of the reach of children. In case of accidental overdose, call a Physician or Poison Control Center immediately.