Product Label for NDC 71756-8050-1 (BZK Towelette)

Directions
Tear open pouch, unfold and use. Discard after single use.
Warnings
- For external use only
- Flammable, keep away from open flame
Inactive Ingredient
purified water, camphor
Do Not Use / Stop Use / Use
(Do not use)
- On irritated skin or over large areas of the body
- If contact occurs with eyes, flush eyes with water
(Stop use)
if irritation and redness develop. Consult your physician if the condition persists.
(Use)
Cleansing of hands, face and body. Air dries in seconds.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center immediately.
Purpose
Antiseptic Cleanser
Active Ingredient
Benzalkonium Chloride 0.13% v/v
Product Label for NDC 71756-8010-1 (Alcohol Prep Pad)
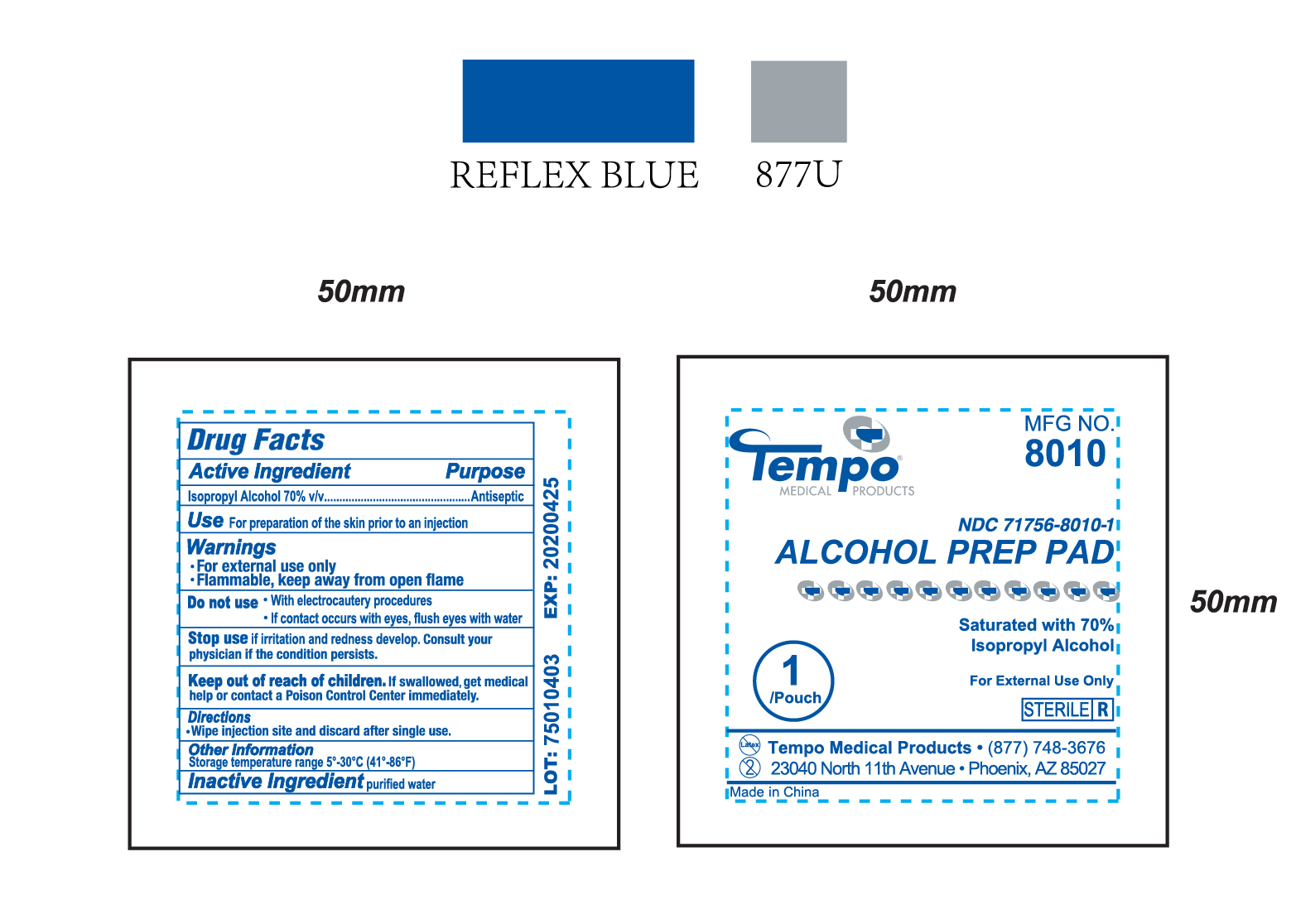
Directions
Wipe injection site and discard after single use.
Warnings
- For external use only
- Flammable, keep away from open flame
Inactive Ingredient
purified water
Do Not Use / Stop Use / Use
(Do not use)
- With electrocautery procedures
- If contact occurs with eyes, flush eyes with water
(Stop use)
if irritation and redness develop. Consult your physician if the condition persists.
(Use)
For preparation of the skin prior to an injection
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center immediately.
Active Ingredient
Isopropyl Alcohol 70% v/v
Product Label for NDC 71756-8090-1 (Sting Relief Pad)
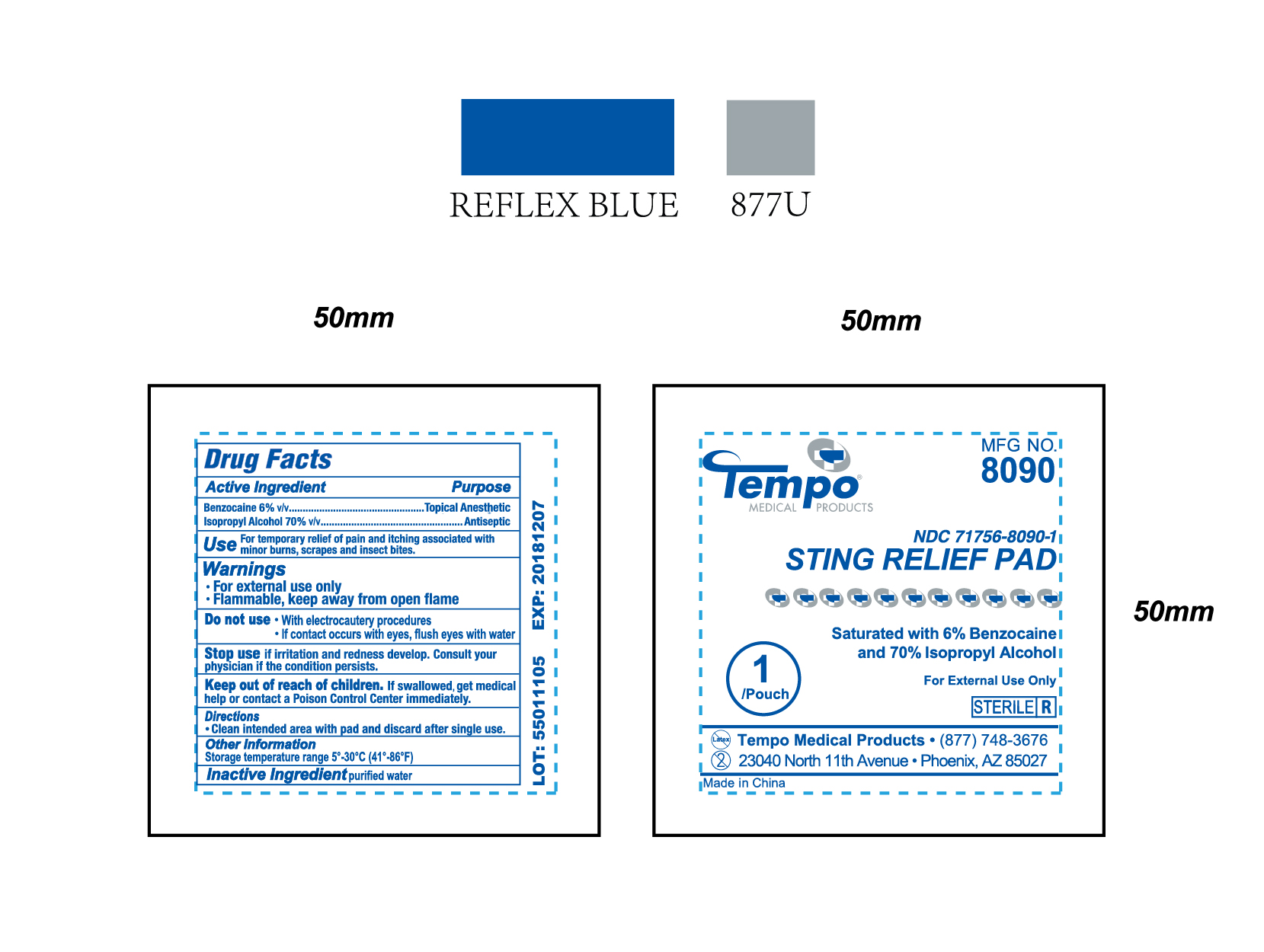
Directions
Clean intended area with pad and discard after single use.
Warnings
- For external use only
- Flammable, keep away from open flame
Inactive Ingredient
purified water
Do Not Use / Stop Use / Use
(Do not use)
- With electrocautery procedures
- If contact occurs with eyes, flush eyes with water
(Stop use)
if irritation and redness develop. Consult your physician if the condition persists.
(Use)
For temporary relief of pain and itching associated with minor burns, scrapes and insect bites.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center immediately.
Purpose
Topical Anesthetic (Benzocaine)
Antiseptic (Isopropyl Alcohol)
Active Ingredient
Benzocaine 6% v/v
Isopropyl Alcohol 70% v/v


