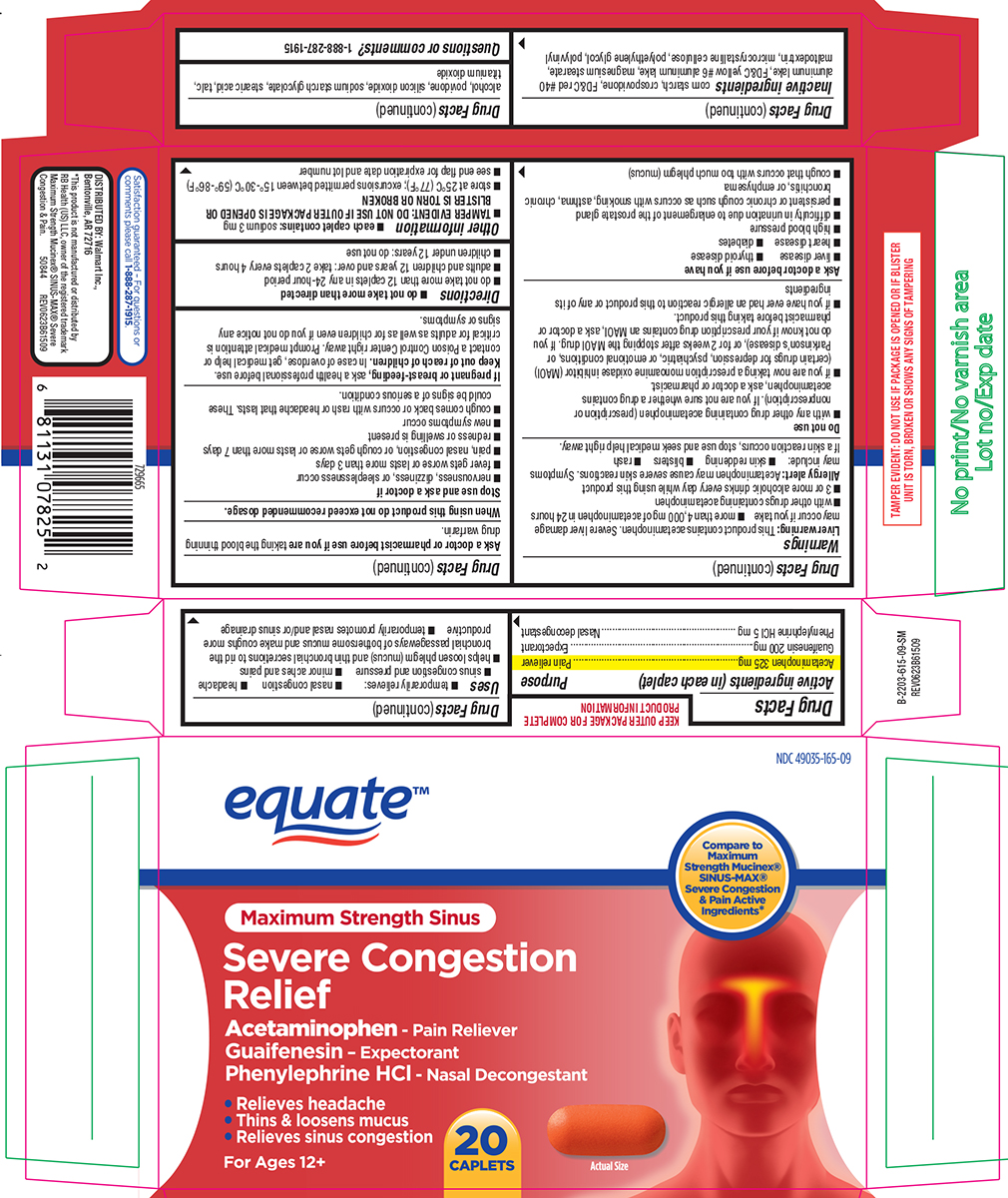
Uses
- temporarily relieves:
- nasal congestion
- headache
- sinus congestion and pressure
- minor aches and pains
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily promotes nasal and/or sinus drainage
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- thyroid disease
- heart disease
- diabetes
- high blood pressure
- difficulty in urination due to enlargement of the prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- fever gets worse or lasts more than 3 days
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
Directions
- do not take more than directed
- do not take more than 12 caplets in any 24-hour period
- adults and children 12 years and over: take 2 caplets every 4 hours
- children under 12 years: do not use
Other information
- each caplet contains: sodium 3 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, crospovidone, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, talc, titanium dioxide
Principal display panel
equate™
NDC 49035-165-09
Compare to
Maximum
Strength Mucinex®
SINUS-MAX®
Severe Congestion
& Pain Active
Ingredients*
Maximum Strength Sinus
Severe Congestion
Relief
Acetaminophen - Pain Reliever
Guaifenesin - Expectorant
Phenylephrine HCl - Nasal Decongestant
• Relieves headache
• Thins & loosens mucus
• Relieves sinus congestion
For Ages 12+
20
CAPLETS
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Satisfaction guaranteed - For questions or
comments please call 1-888-287-1915
DISTRIBUTED BY: Walmart Inc.,
Bentonville, AR 72716
*This product is not manufactured or distributed by
RB Health (US) LLC, owner of the registered trademark
Maximum Strength Mucinex® SINUS-MAX® Severe
Congestion & Pain. 50844 REV0623B61509
Equate 44-615