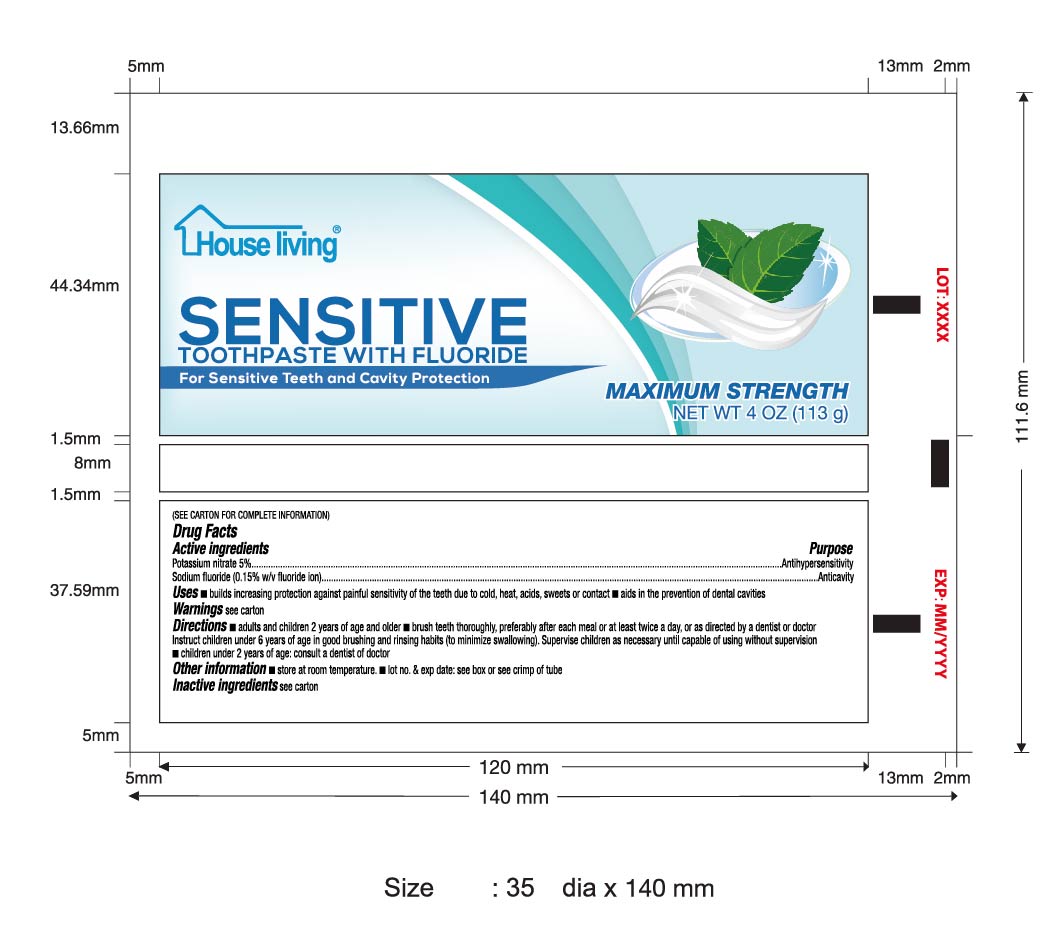
Use
bulids increasing protection against painful sensitivity of the teeth due to cold,heat,acids,sweets or contact
aids in the privention of dental cavities
Warning
Stop use and ask a dentist if
the problem pensists os worsens,sensitive teeth may indicate a serious problem that may prompt care by a dentist
pain/sensitivity still persists after 4 weeks of use.
Directions
Adults and children 2 years of age and older:brush teeth thoroughly, preferably after meals or at least twice a day or use as directed by a dentist or doctor.
instruct children 6 years of age in good brushing and rinsing habits(to minimize swallowing)
suprevise children as necessary untill capable of using without supervision.
children under 2 years of age :consult a dentist or doctor
Inactive Ingredients
Water,Silica (Thickening 10 & Friction 4)
,Sodium Lauryl Alcohol,Sorbitol,Polyethylene Glycol 300,Sodium Carboxymethylcellulose,Flavor,Sodium Saccharin,Titanium Dioxide,Sodium Pyrophosphate,PEG/PPG 116/66 copolymer
Sodium Hydroxide,Acid Yellow 23,Acid Blue 1