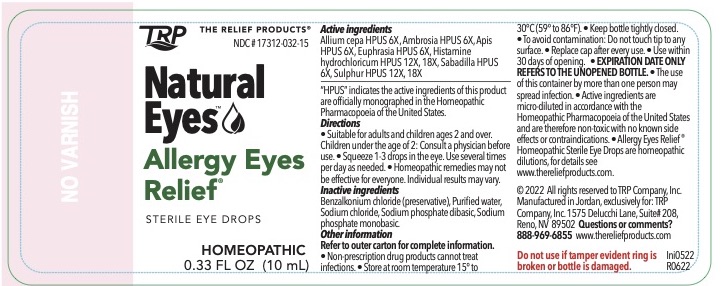
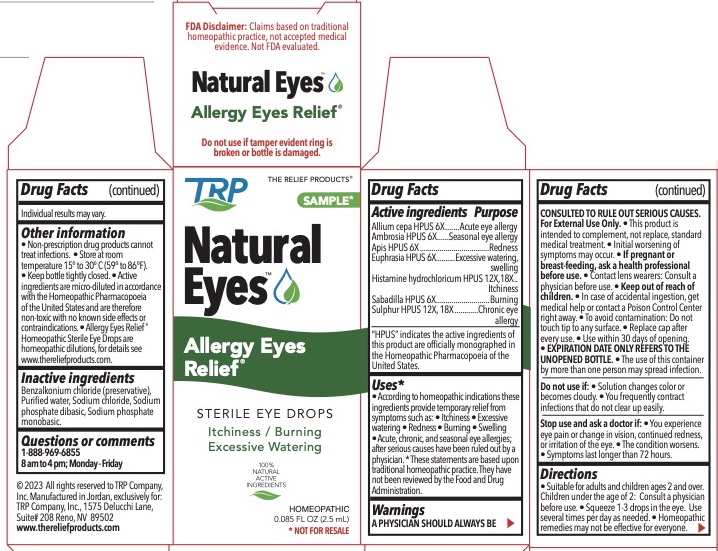
| Active Ingredients | Purpose |
| Allium cepa HPUS 6X | Acute eye allergy |
| Ambrosia HPUS 6X | Seasonal eye allergy |
| Euphrasia HPUS 6X | Redness |
| Histamine hydrochloricum HPUS 12X, 18X | Itchiness |
| Sabadilla HPUS 6X | Burning |
| Sulphur HPUS 6X, 12X, 18X | Burning |
"HPUS" indicates the active ingredients are in the Homeopathic Pharmacopoeia of the United States.
Uses:*
According to homeopathic indiciations these ingredients provide temporary relief from symptoms such as: • Itchiness • Excessive watering • Redness • Burning • Swelling • Acute, chronic, and seasonal eye allergies after serious causes have been ruled out by a physician.
* These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
Warnings:
A PHYSICIAN SHOULD ALWAYS BE CONSULTED TO RULE OUT SERIOUS CAUSES. For External Use Only. • This product is intended to complement, not replace, standard medical treatment. • Initial worsening of symptoms may occur. • If pregnant or breast-feeding, ask a health professional before use. Contact lens wearers: Consult a physician before use. • Keep out of reach of children. • In case of accidental ingestion, get medical help or contact a Poison Control Center right away. • To avoid contamination – do not touch tip to any surface. • Replace cap after every use. • Use within 30 days of opening. • EXPIRATION DATE ONLY REFERS TO UNOPENED BOTTLE. • The use of this container by more than one person may spread infection.
Do not use:
- If solution changes color or becomes cloudy.
- If you frequently contract infections that do not clear up easily.
- If you experience eye pain or changes in vision.
Stop use and ask a doctor if:
- You experience eye pain, changes in vision, continued redness or irritation of the eye.
- The condition worsens.
- The condition persists for more than 72 hours.
Keep out of reach of children
• In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Directions:
• Suitable for adults and children ages 2 and over. Children under the age of 2: Consult a physician before use. • Squeeze 1-3 drops in the eye. Use several times per day as needed. • Homeopathic remedies may not be effective for everyone. Individual results may vary.
Other information:
- There are no known contraindications. • Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no known side effects. • Store at room temperature 15° to 30°C (59° to 86°F). • Keep bottle tightly closed. Allergy Eyes Relief
TM Homeopathic Sterile Eye Drops are homeopathic dilutions, for details see www.thereliefproducts.com.