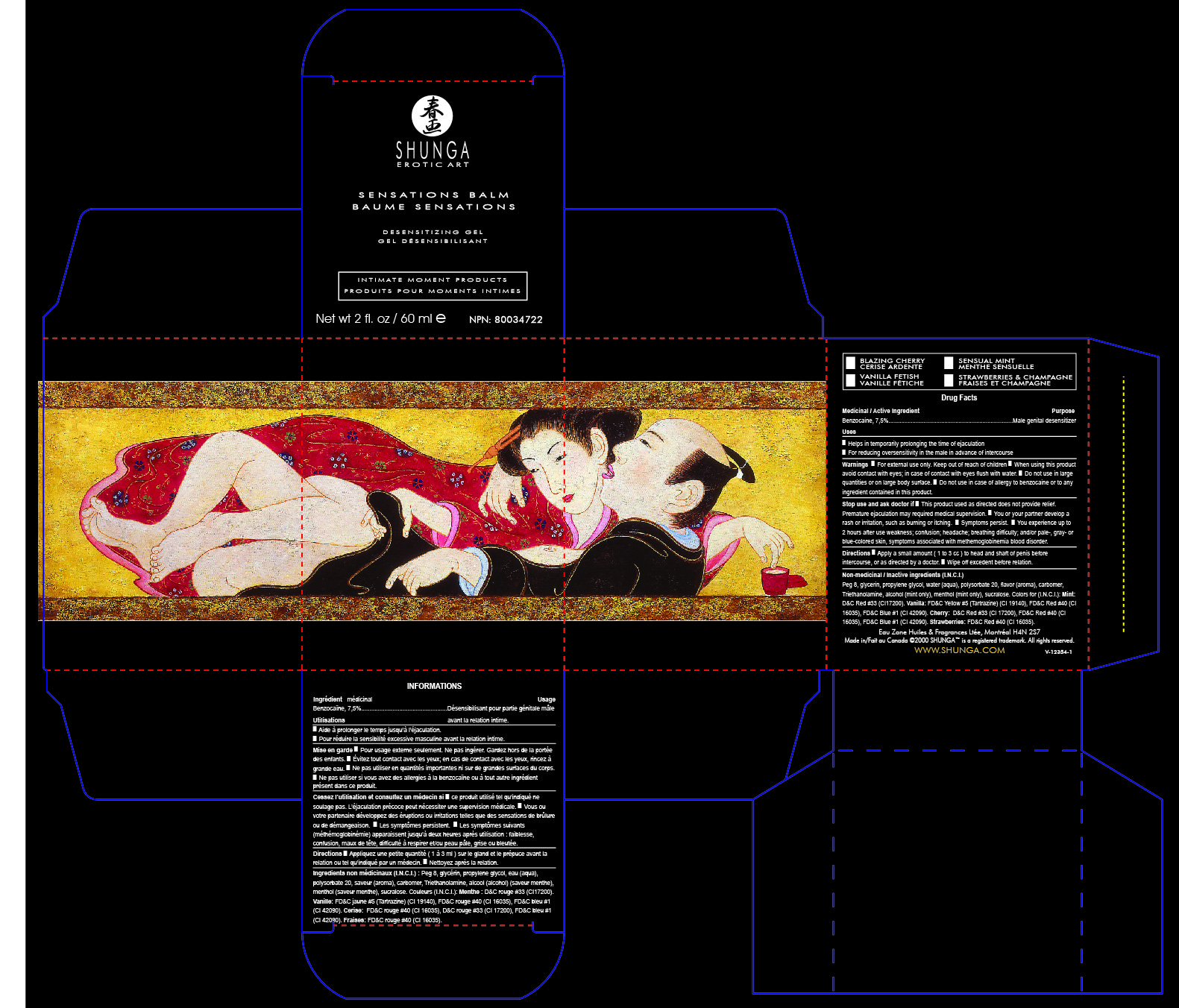
Active ingredient
Male Desensitizing Gel
Gel désensibilisant mâle
Active Ingredient
Benzocaine 7.5 pourcentage
Genital Desensitizer
Désensibilisant Genital
Benzocaine USP 7.5 pourcent
60ml
Warnings
■ For external use only. Keep out of reach of children ■ When using this product
avoid contact with eyes; in case of contact with eyes flush with water. ■ Do not use in large
quantities or on large body surface. ■ Do not use in case of allergy to benzocaine or to any
ingredient contained in this product.
Directions
Directions ■ Apply a small amount ( 1 to 3 cc ) to head and shaft of penis before
intercourse, or as directed by a doctor. ■ Wipe off excedent before relation.
Stop use and ask doctor if
Stop use and ask doctor if ■ This product used as directed does not provide relief.
Premature ejaculation may required medical supervision. ■ You or your partner develop a
rash or irritation, such as burning or itching. ■ Symptoms persist. ■ You experience up to
2 hours after use weakness; confusion; headache; breathing difficulty; and/or pale-, gray- or
blue-colored skin, symptoms associated with methemoglobinemia blood disorder.
Warning
Warnings
For external use only. Keep out of reach of children
When using this product avoid contact with eyes; in case of contact with eyes flush with water.
Do not use in large quantities or on large body surface.
Do not use in case of allergy to benzocaine or to any ingredient contained in this product.
Purpose
Name: SHUNGA Sensation Balm
SHUNGA Male Genital Desensitizer helps in temporarily prolonging the time until ejaculation by diminishing tactile sensations to the penis.
Purpose
Male genital desensitizer
Uses
Helps in temporarily prolonging the time of ejaculation ■ For reducing oversensitivity in the male in advance of intercourse
Directions ■ Apply a small amount ( 1 to 3 cc ) to head and shaft of penis before
intercourse, or as directed by a doctor. ■ Wipe off excedent before relation.
Non-medicinal / Inactive ingredients (I.N.C.I.)
Non-medicinal / Inactive ingredients (I.N.C.I.)
Water (aqua), peg 8, glycerin, propylene glycol, triethanolamine, carbomer, flavor (aroma),
sucralose. Colors for (I.N.C.I.): Mint : D&C Red #33 (CI17200). Vanilla: FD&C Yellow #5
(Tartrazine) (CI 19140), FD&C Red #40 (CI 16035), FD&C Blue #1 (CI 42090). Cherry and
Strawberries: FD&C Red #40 (CI 16035), D&C Red #33 (CI 17200).
Eau Zone Huiles & Fragrances Ltée, Montréal H4N 2S7
Made in/Fait au Canada ©2000 SHUNGA™ is a registered trademark. All rights reserved.